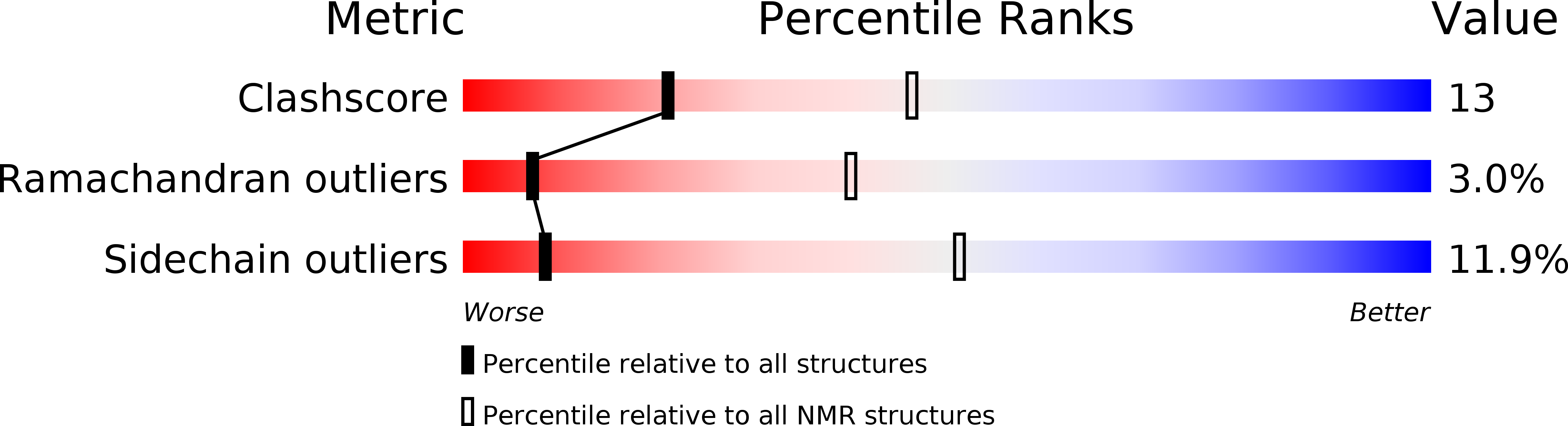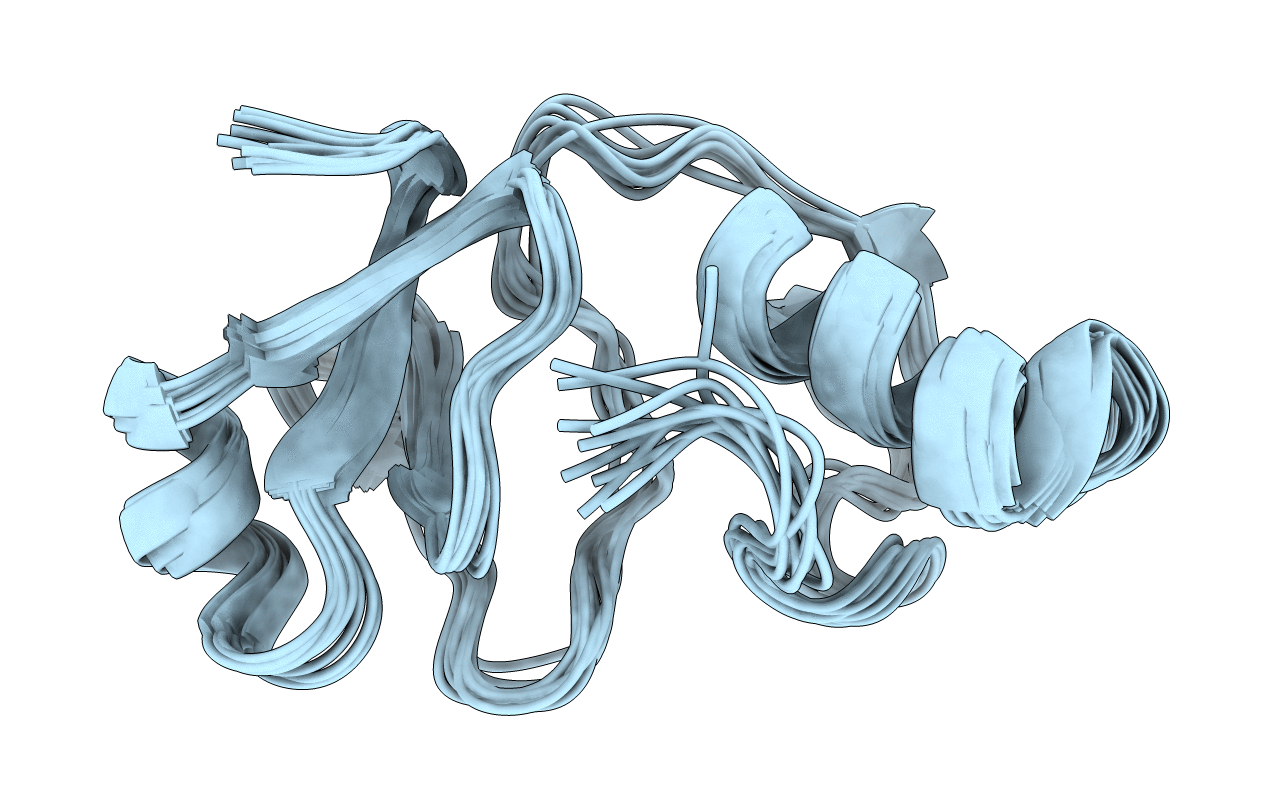
Deposition Date
2018-12-21
Release Date
2019-12-11
Last Version Date
2025-04-09
Entry Detail
PDB ID:
6QBL
Keywords:
Title:
NMR Structure of Big-defensin 1 from oyster Crassostrea gigas
Biological Source:
Source Organism(s):
Crassostrea gigas (Taxon ID: 29159)
Method Details:
Experimental Method:
Conformers Calculated:
150
Conformers Submitted:
10
Selection Criteria:
total energies and restraint violation statistics