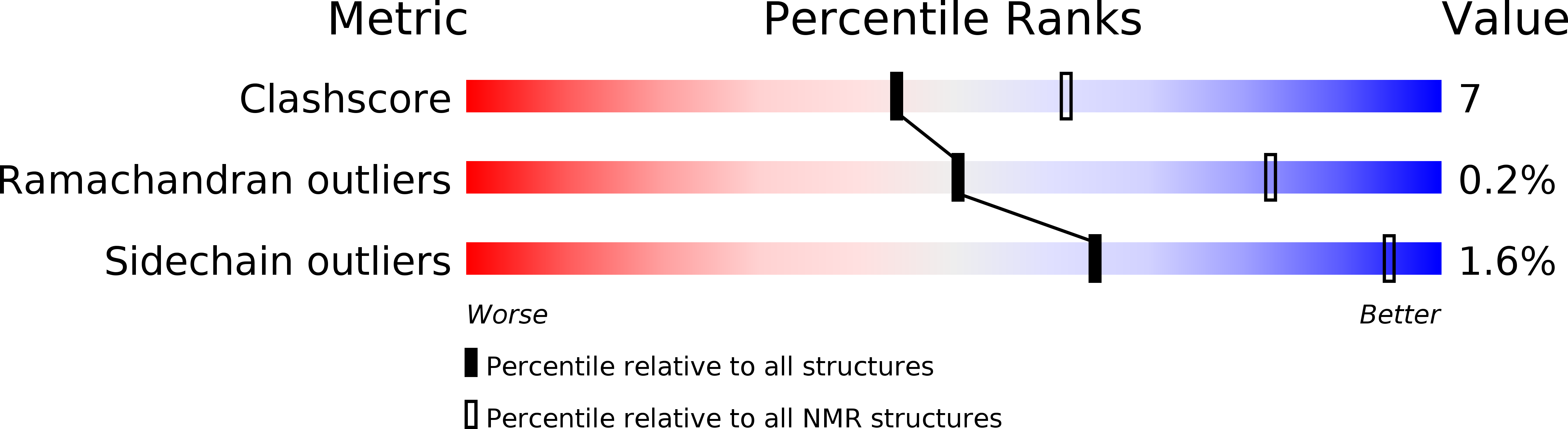
Deposition Date
2018-12-21
Release Date
2020-01-29
Last Version Date
2024-11-06
Entry Detail
PDB ID:
6QBI
Keywords:
Title:
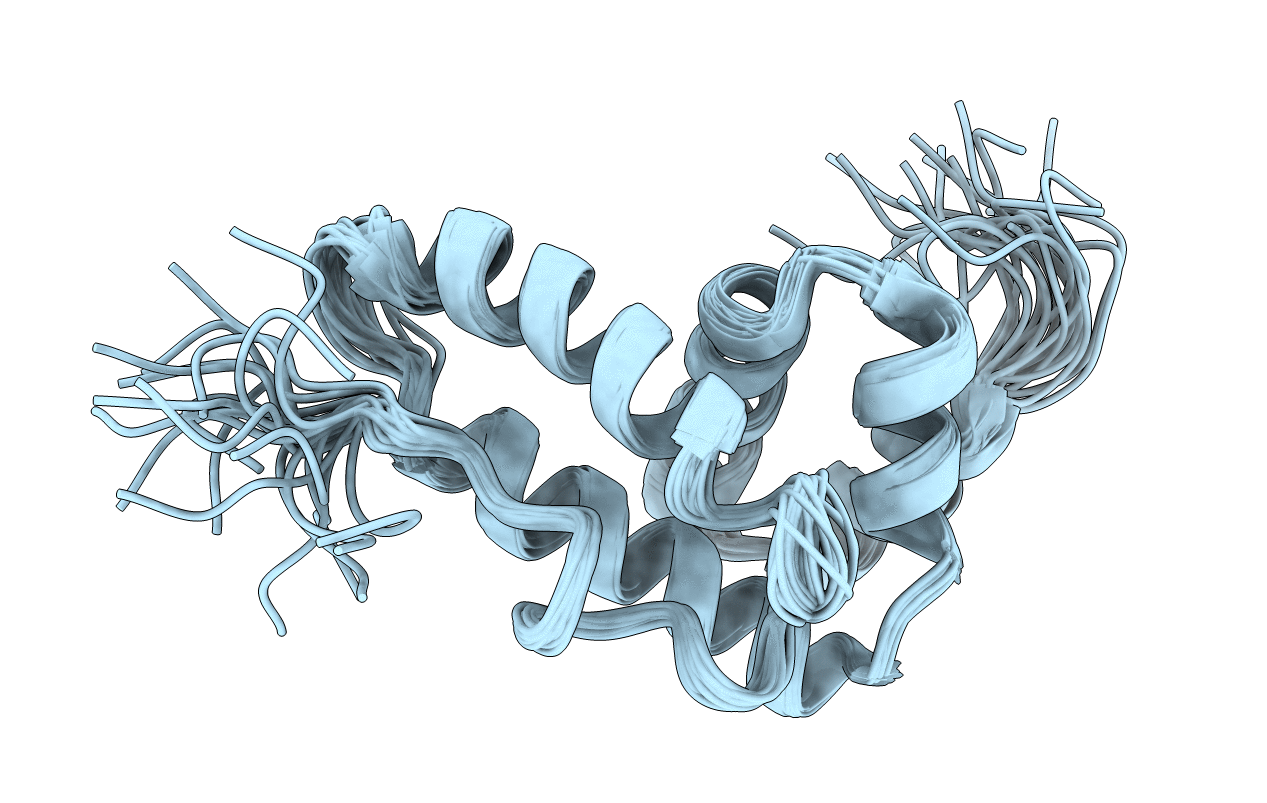
NMR structure of BB_P28, Borrelia burgdorferi outer surface lipoprotein
Biological Source:
Source Organism:
Host Organism:
Method Details:
Experimental Method:
Conformers Calculated:
100
Conformers Submitted:
20
Selection Criteria:
structures with the least restraint violations