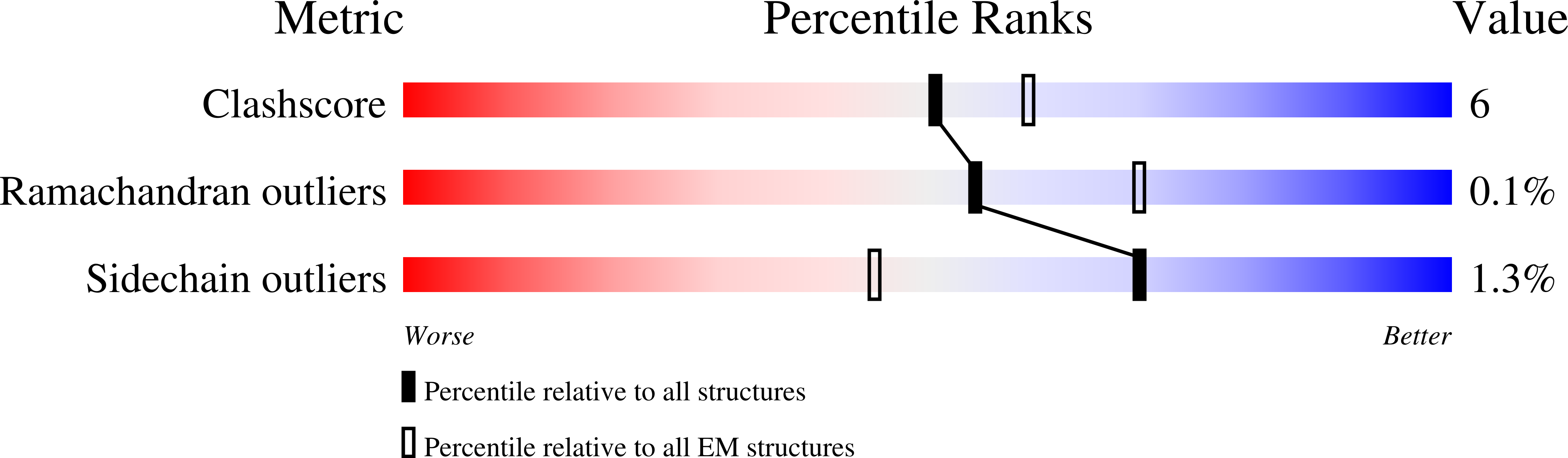
Deposition Date
2018-12-17
Release Date
2019-08-21
Last Version Date
2024-11-20
Entry Detail
PDB ID:
6Q9B
Keywords:
Title:
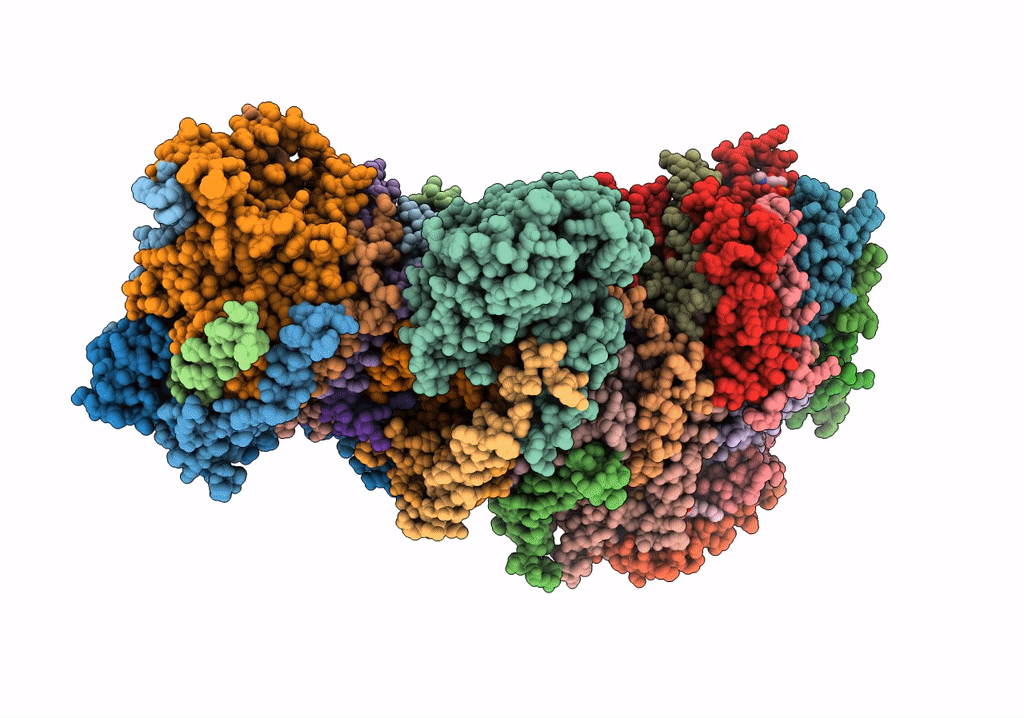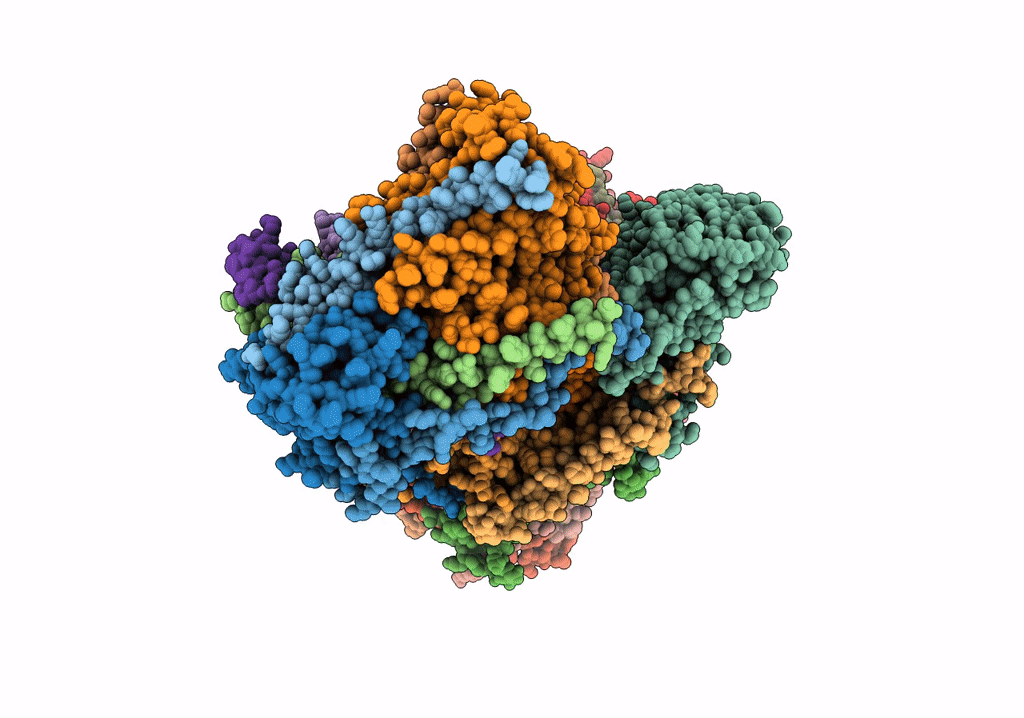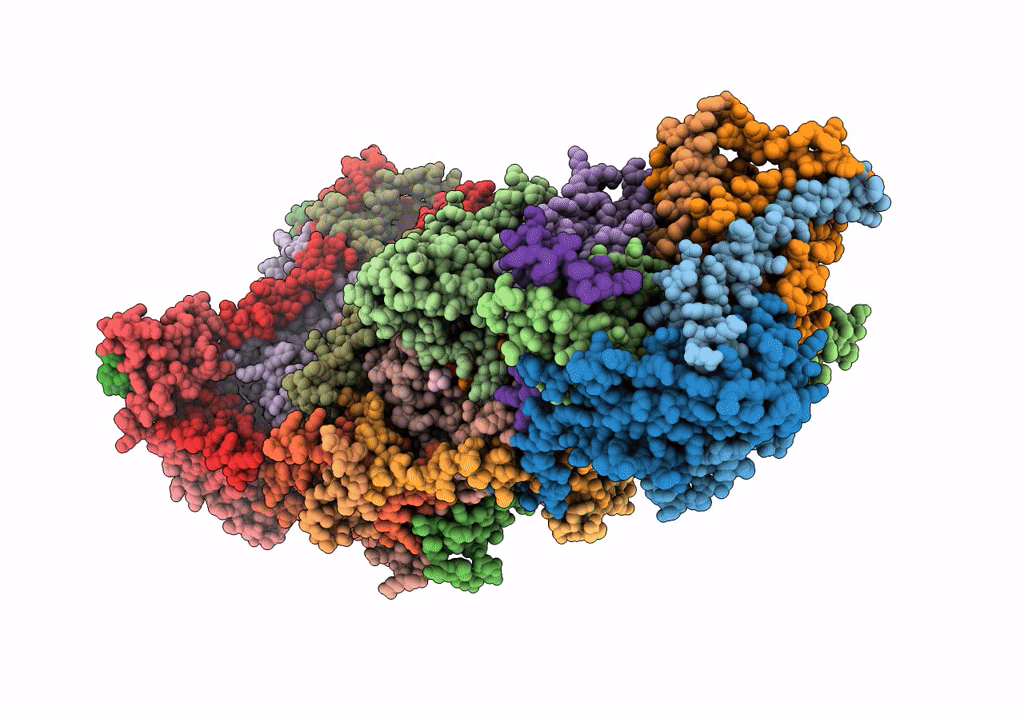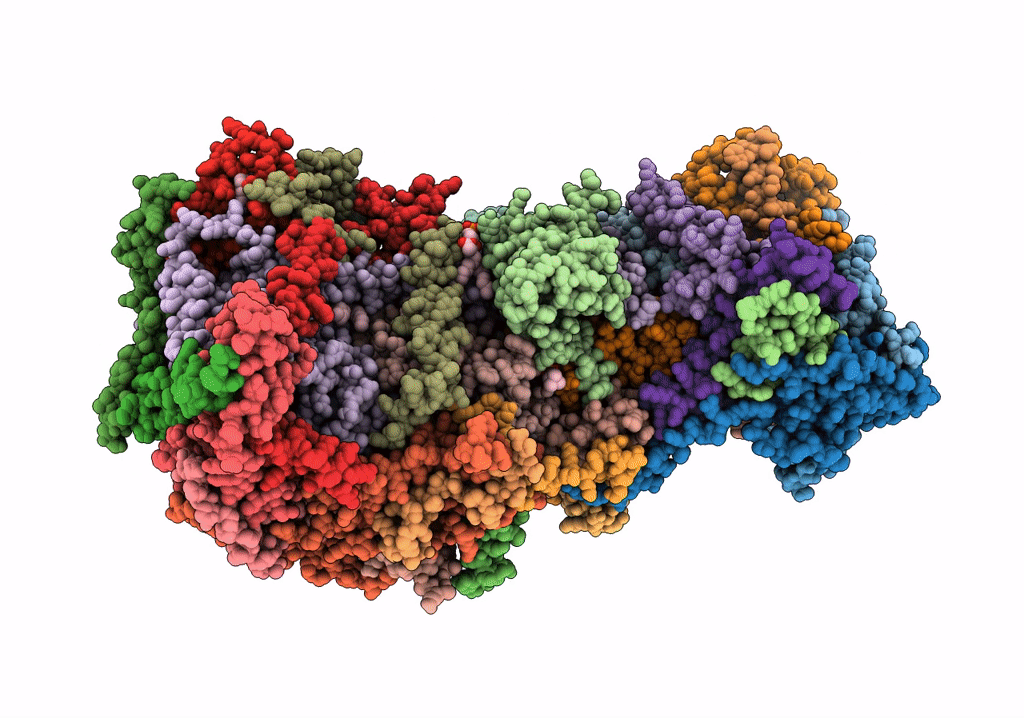
CI Membrane Arm focused refinement from Ovine respiratory SC I+III2
Biological Source:
Source Organism(s):
Ovis aries (Taxon ID: 9940)
Method Details:
Experimental Method:
Resolution:
3.90 Å
Aggregation State:
PARTICLE
Reconstruction Method:
SINGLE PARTICLE