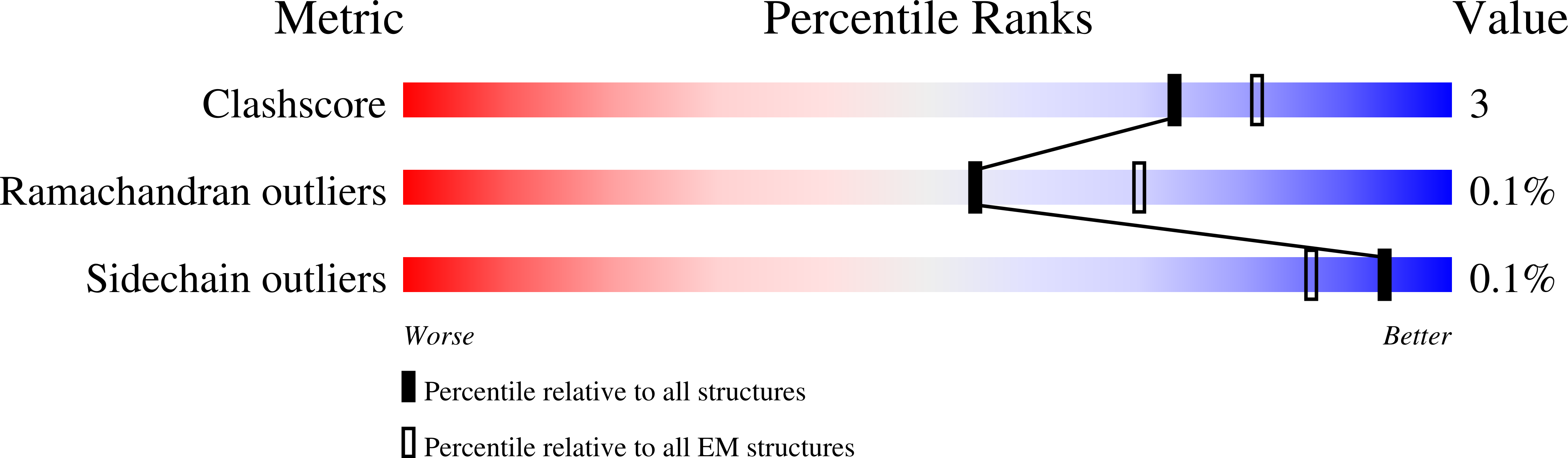
Deposition Date
2018-12-09
Release Date
2019-06-19
Last Version Date
2024-05-15
Entry Detail
PDB ID:
6Q5U
Keywords:
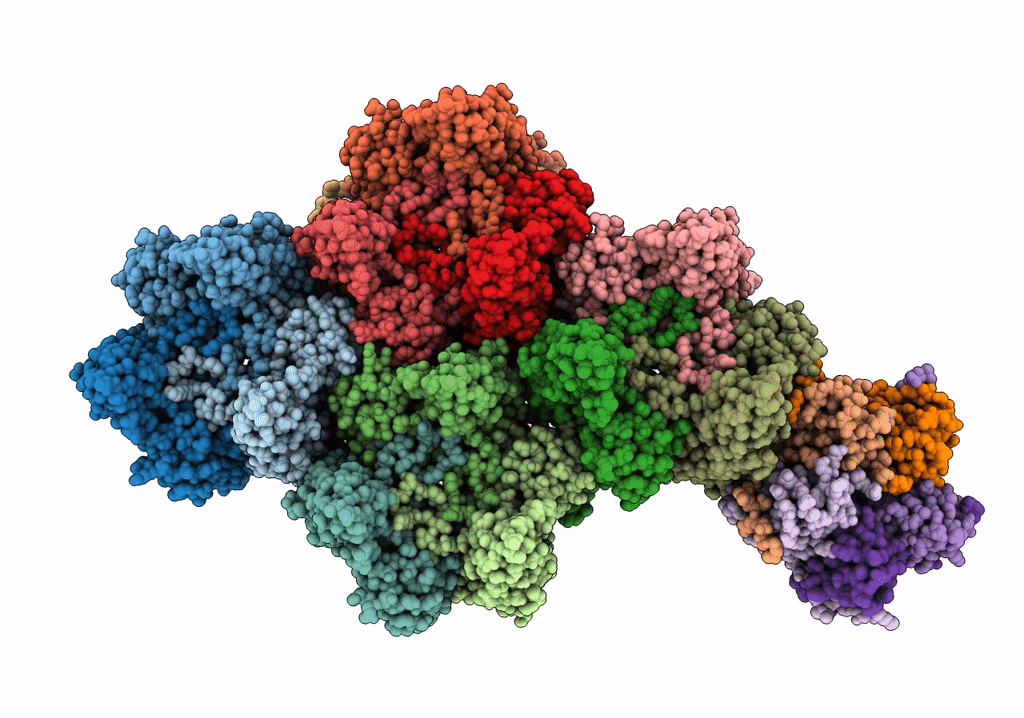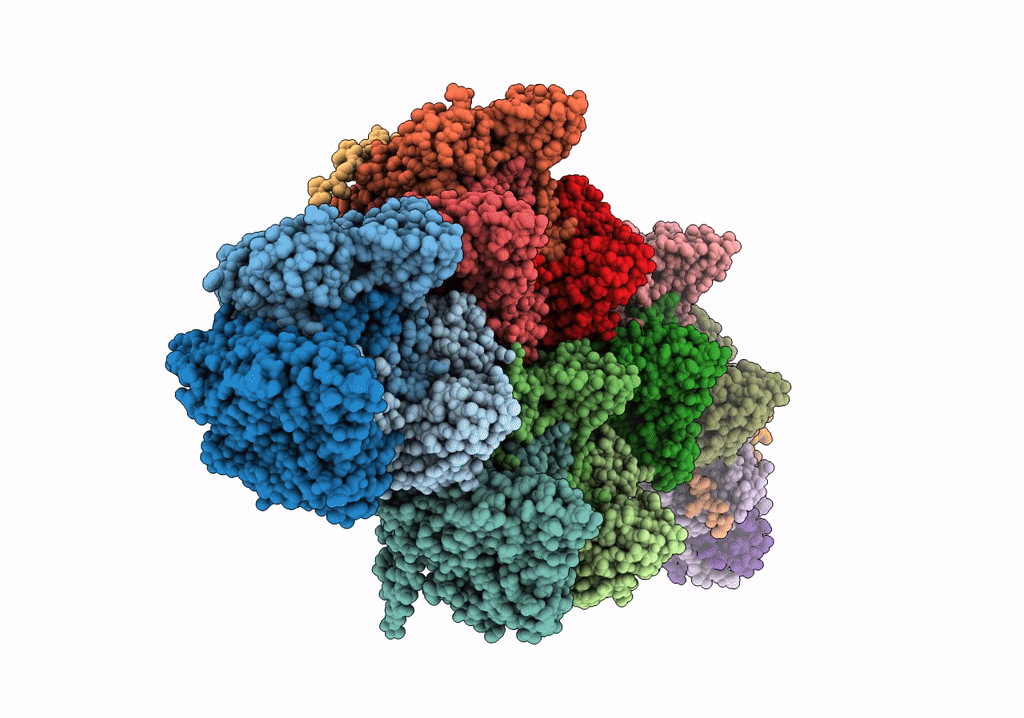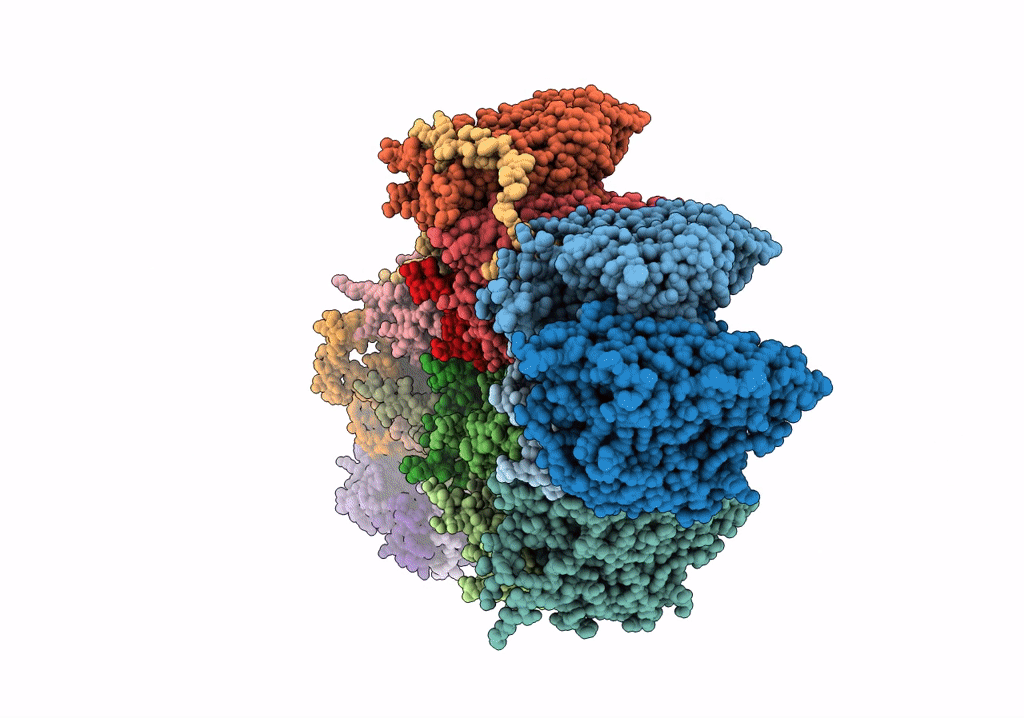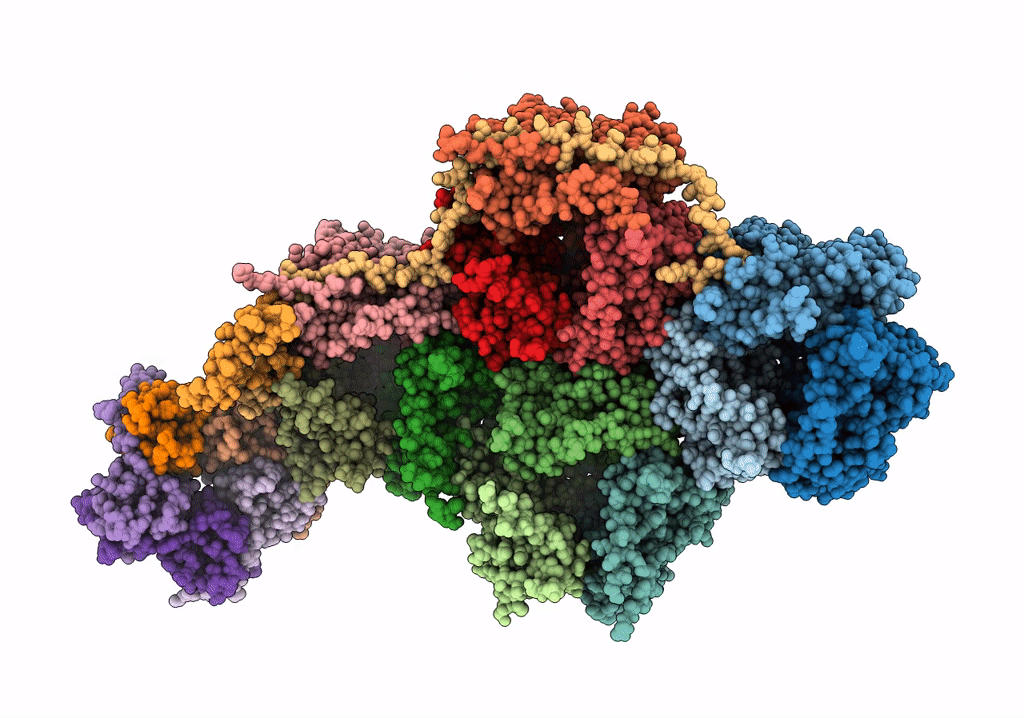
Title:
High resolution electron cryo-microscopy structure of the bacteriophage PR772
Biological Source:
Source Organism:
Enterobacteria phage PR772 (Taxon ID: 261665)
Method Details:
Experimental Method:
Resolution:
2.75 Å
Aggregation State:
PARTICLE
Reconstruction Method:
SINGLE PARTICLE