Deposition Date
2018-12-09
Release Date
2019-05-22
Last Version Date
2024-10-16
Entry Detail
PDB ID:
6Q5M
Keywords:
Title:
Crystal structure of a CC-Hex mutant that forms an antiparallel four-helix coiled coil CC-Hex*-L24Dab
Biological Source:
Source Organism(s):
synthetic construct (Taxon ID: 32630)
Method Details:
Experimental Method:
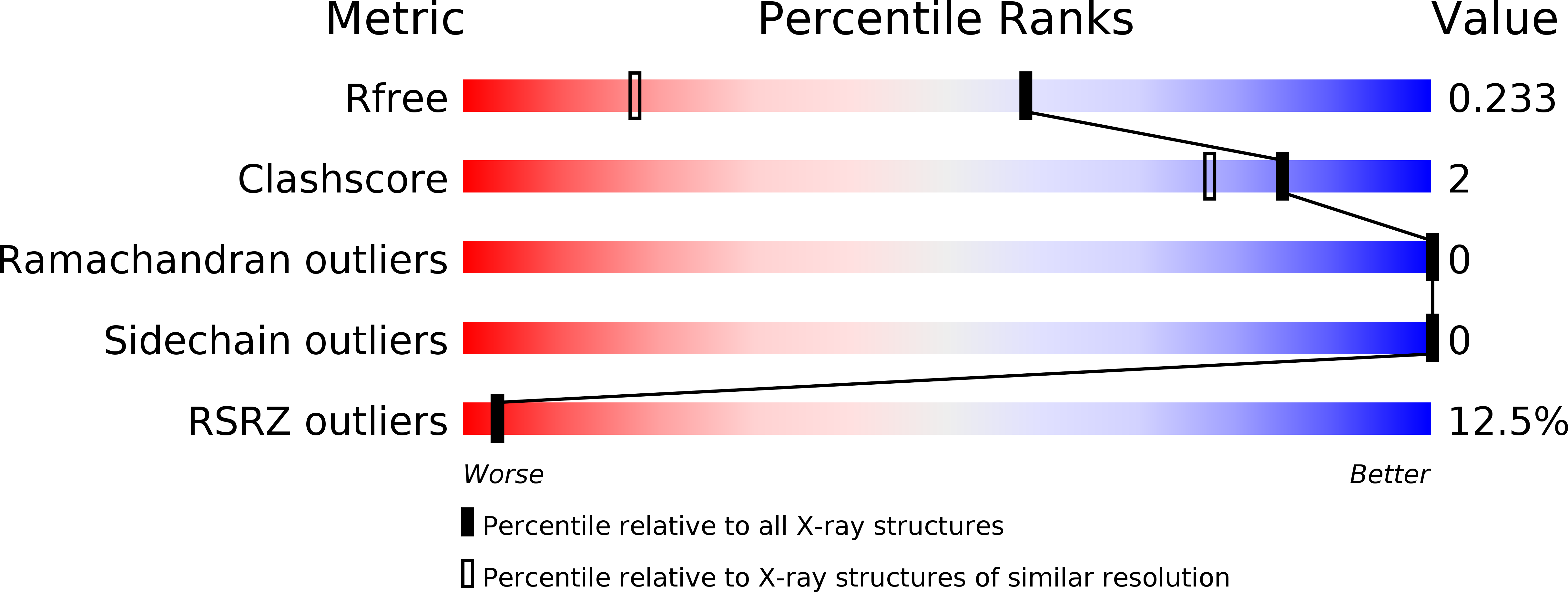
Resolution:
1.50 Å
R-Value Free:
0.22
R-Value Work:
0.19
R-Value Observed:
0.19
Space Group:
P 61 2 2