Deposition Date
2018-12-06
Release Date
2019-09-18
Last Version Date
2024-01-24
Entry Detail
PDB ID:
6Q4Y
Keywords:
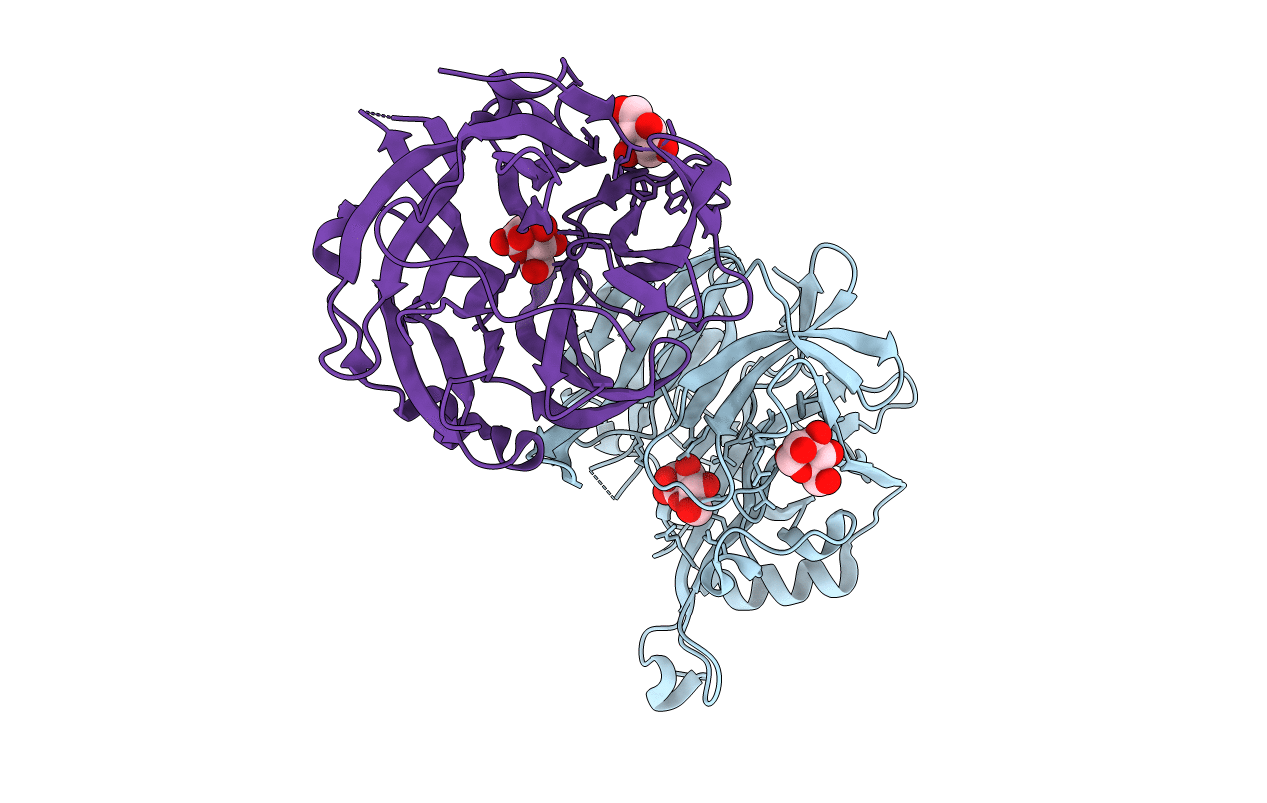
Title:
Structure of MPT-2, a GDP-Man-dependent mannosyltransferase from Leishmania mexicana, in complex with mannose
Biological Source:
Source Organism:
Leishmania mexicana MHOM/GT/2001/U1103 (Taxon ID: 929439)
Host Organism:
Method Details:
Experimental Method:
Resolution:
1.70 Å
R-Value Free:
0.21
R-Value Work:
0.17
R-Value Observed:
0.17
Space Group:
P 21 21 21


