Deposition Date
2019-07-28
Release Date
2019-10-09
Last Version Date
2024-11-20
Entry Detail
PDB ID:
6PXX
Keywords:
Title:
Class D beta-lactamase in complex with beta-lactam antibiotic
Biological Source:
Source Organism:
Klebsiella pneumoniae (Taxon ID: 573)
Host Organism:
Method Details:
Experimental Method:
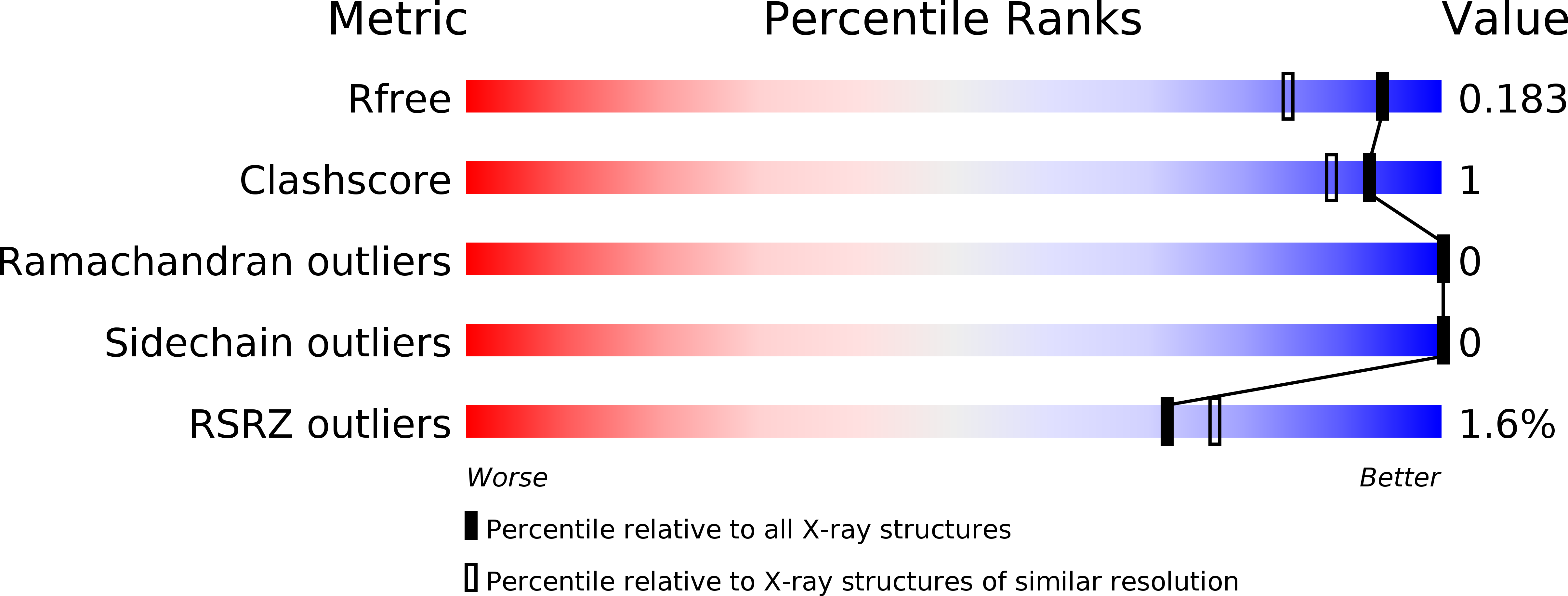
Resolution:
1.50 Å
R-Value Free:
0.17
R-Value Work:
0.15
R-Value Observed:
0.15
Space Group:
P 21 21 2