Deposition Date
2019-07-22
Release Date
2020-03-25
Last Version Date
2024-03-20
Entry Detail
PDB ID:
6PWB
Keywords:
Title:
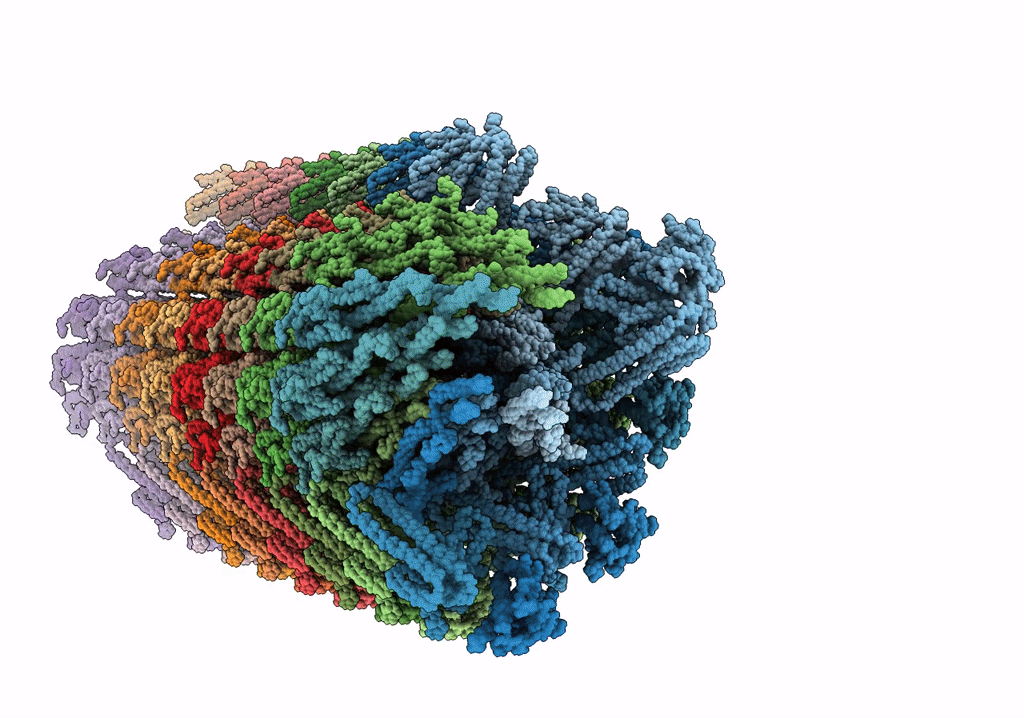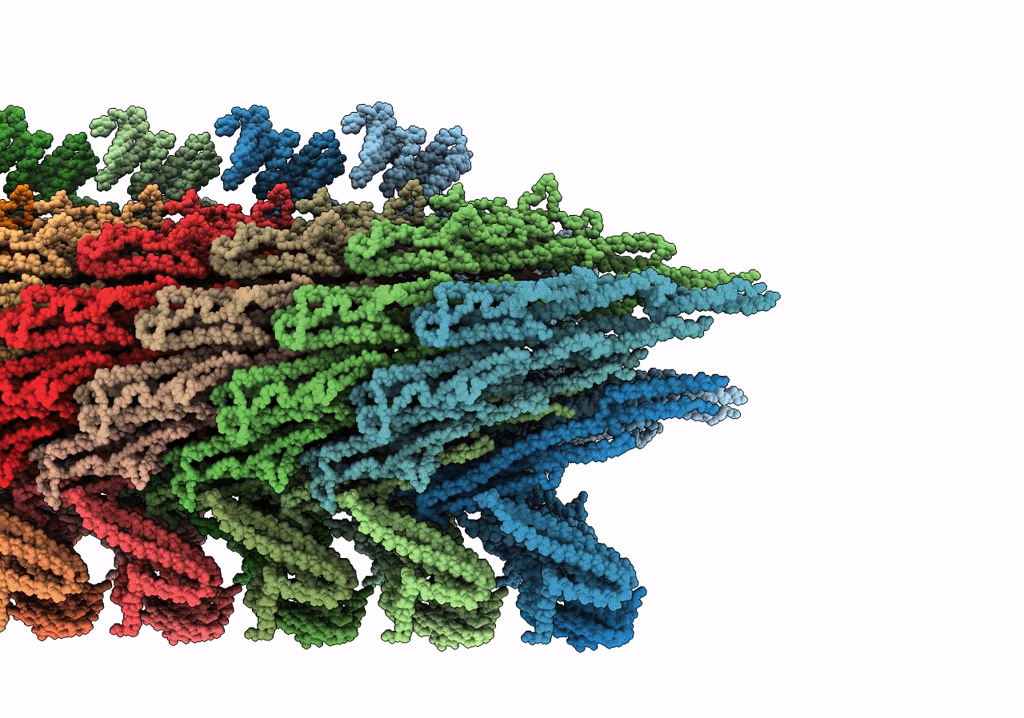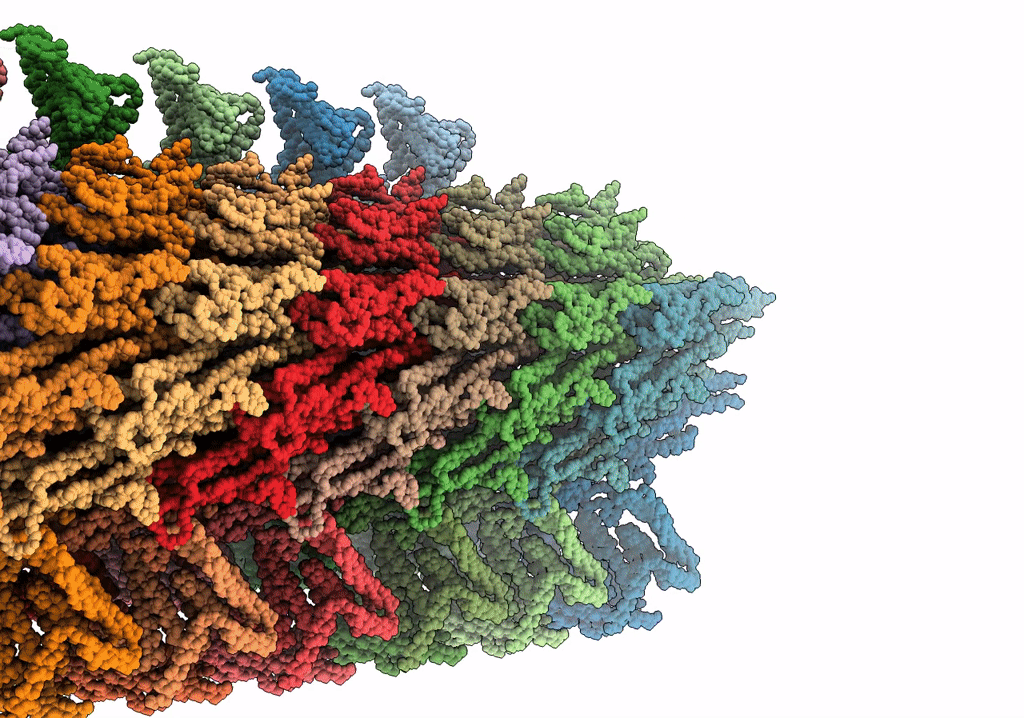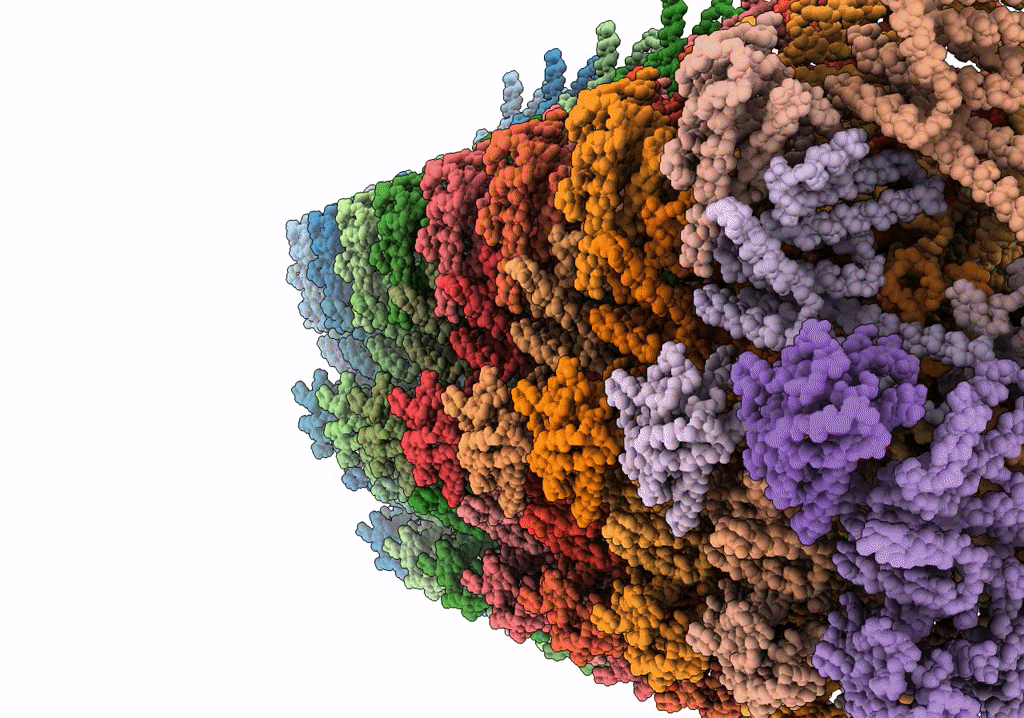
Rigid body fitting of flagellin FlaB, and flagellar coiling proteins, FcpA and FcpB, into a 10 Angstrom structure of the asymmetric flagellar filament purified from Leptospira biflexa Patoc WT cells resolved via subtomogram averaging
Biological Source:
Source Organism(s):
Method Details:
Experimental Method:
Resolution:
9.83 Å
Aggregation State:
FILAMENT
Reconstruction Method:
SUBTOMOGRAM AVERAGING