Deposition Date
2019-07-01
Release Date
2020-04-29
Last Version Date
2024-05-15
Entry Detail
PDB ID:
6PMG
Keywords:
Title:
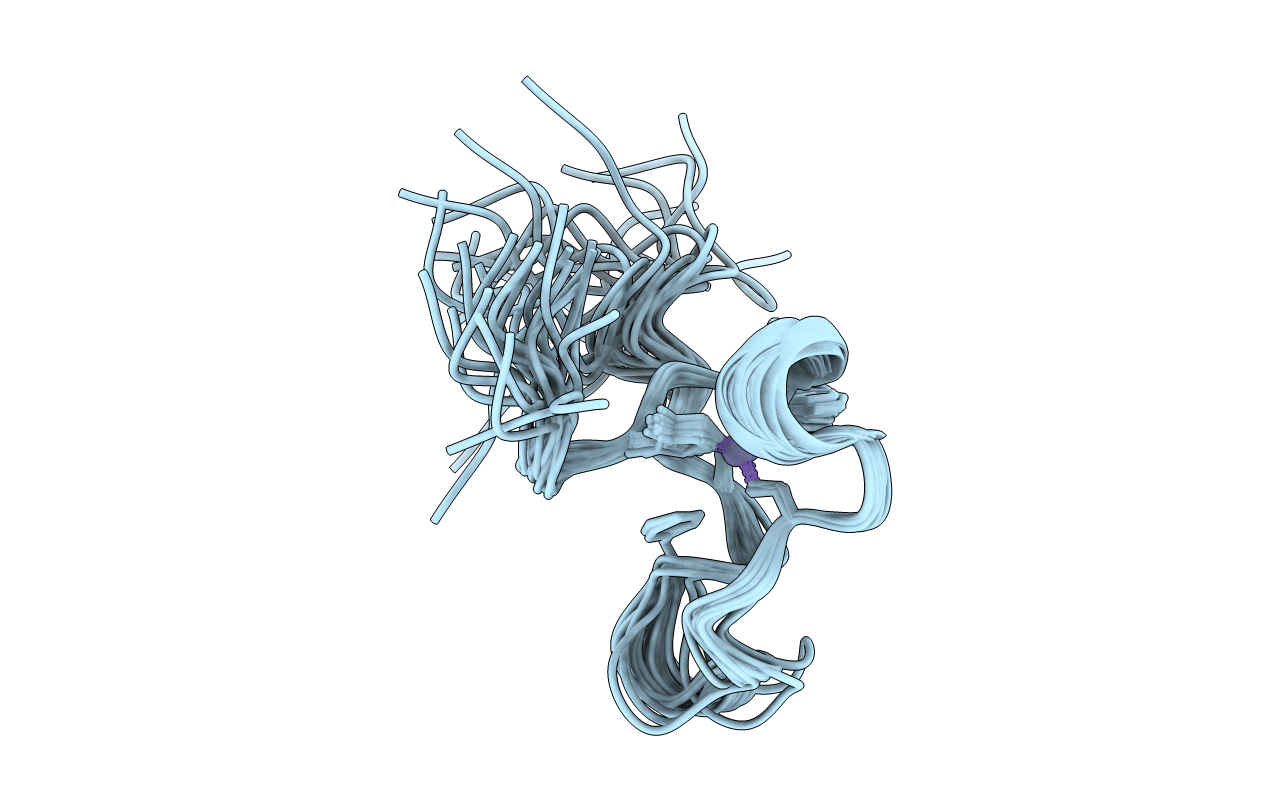
Solution structure of the C-terminal zinc finger of the C. elegans protein MEX-5
Biological Source:
Source Organism(s):
Caenorhabditis elegans (Taxon ID: 6239)
Expression System(s):
Method Details:
Experimental Method:
Conformers Calculated:
10
Conformers Submitted:
20
Selection Criteria:
all calculated structures submitted


