Deposition Date
2019-06-13
Release Date
2019-10-23
Last Version Date
2023-10-11
Entry Detail
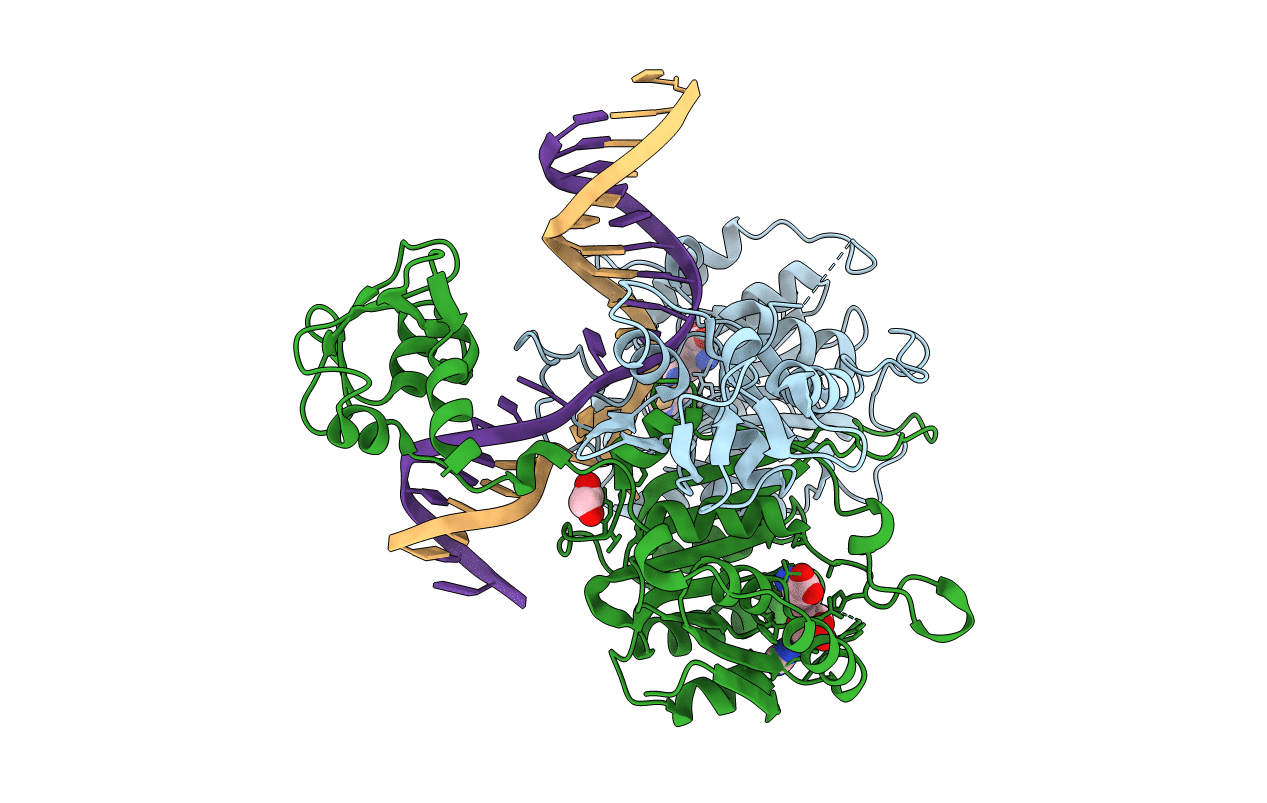
PDB ID:
6PBD
Keywords:
Title:
DNA N6-Adenine Methyltransferase CcrM In Complex with Double-Stranded DNA Oligonucleotide Containing Its Recognition Sequence GAATC
Biological Source:
Source Organism(s):
Caulobacter vibrioides (Taxon ID: 155892)
synthetic construct (Taxon ID: 32630)
synthetic construct (Taxon ID: 32630)
Expression System(s):
Method Details:
Experimental Method:
Resolution:
2.34 Å
R-Value Free:
0.20
R-Value Work:
0.17
R-Value Observed:
0.17
Space Group:
P 21 21 21


