Deposition Date
2019-06-06
Release Date
2019-08-21
Last Version Date
2023-08-16
Entry Detail
PDB ID:
6P7N
Keywords:
Title:
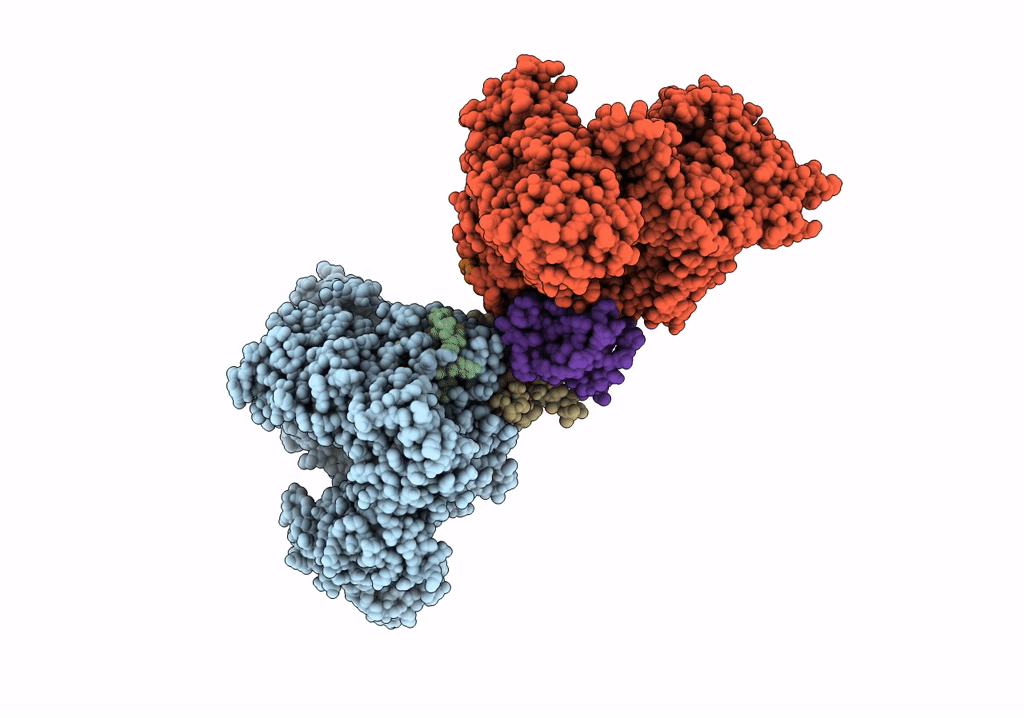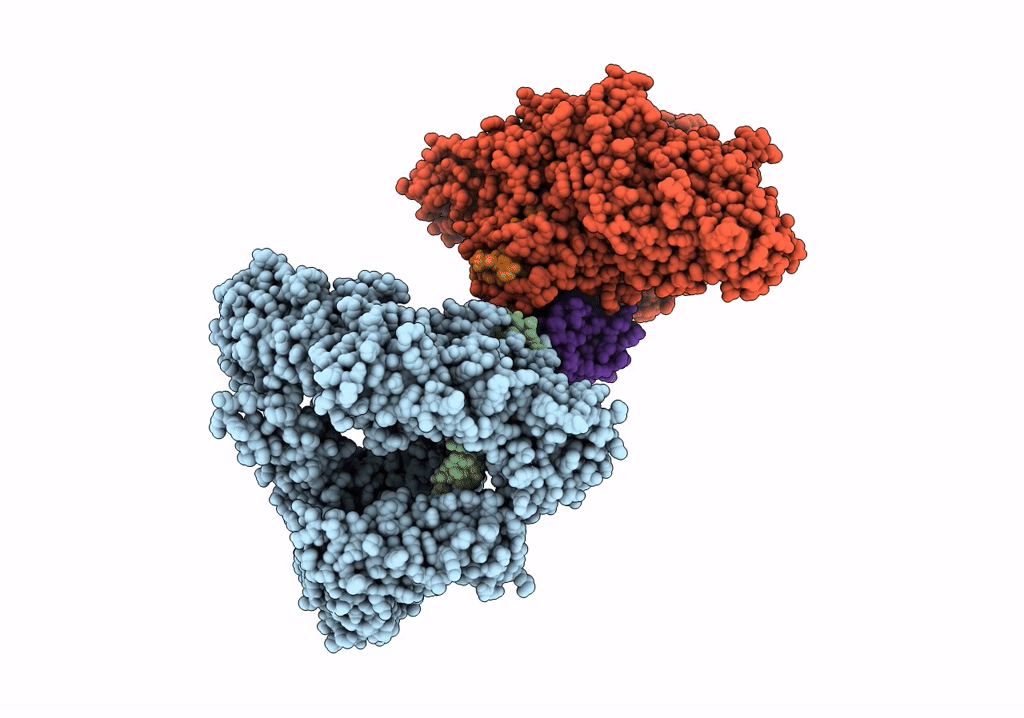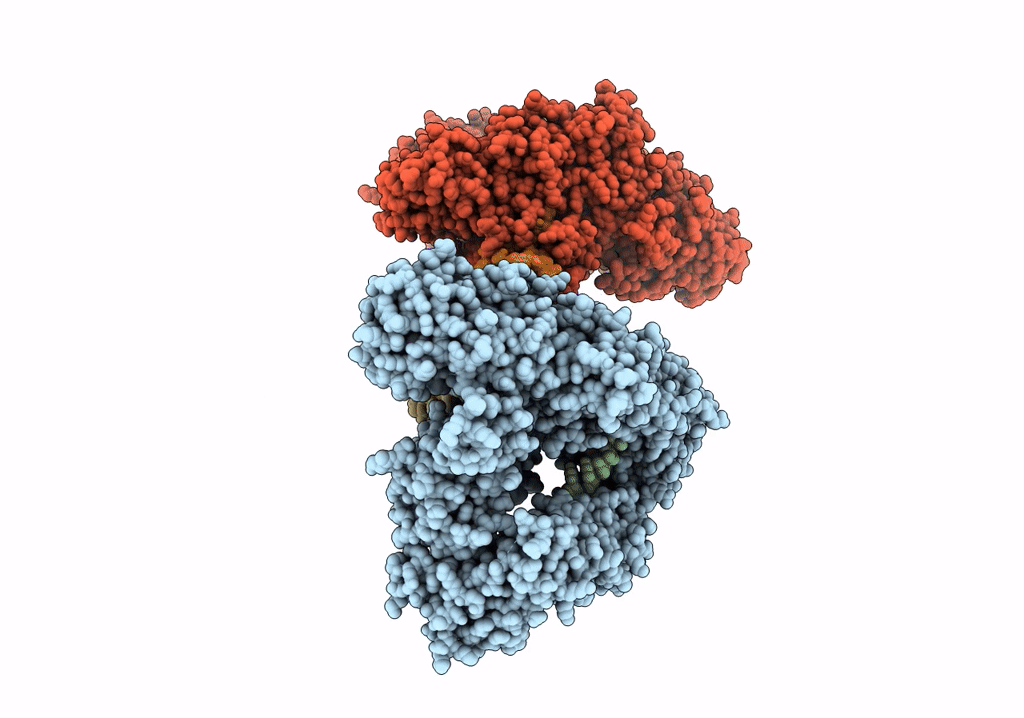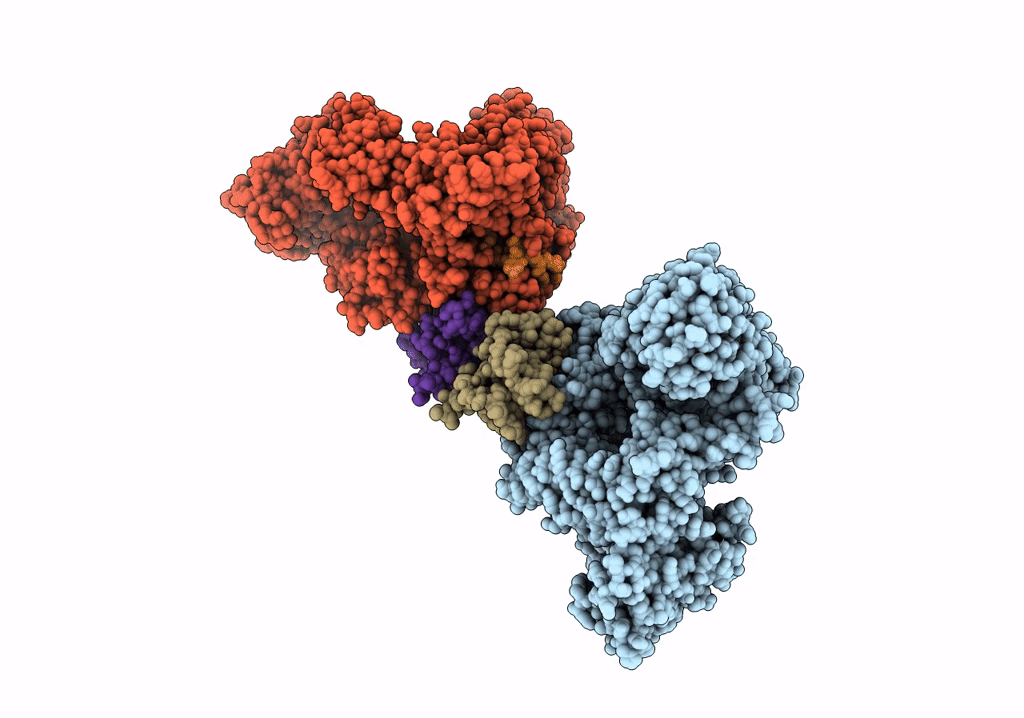
Cryo-EM structure of LbCas12a-crRNA: AcrVA4 (2:2 complex)
Biological Source:
Source Organism(s):
Moraxella bovoculi (Taxon ID: 386891)
Lachnospiraceae bacterium ND2006 (Taxon ID: 1410628)
Lachnospiraceae bacterium ND2006 (Taxon ID: 1410628)
Expression System(s):
Method Details:
Experimental Method:
Resolution:
4.90 Å
Aggregation State:
PARTICLE
Reconstruction Method:
SINGLE PARTICLE


