Deposition Date
2019-06-05
Release Date
2020-03-04
Last Version Date
2023-10-11
Entry Detail
PDB ID:
6P7E
Keywords:
Title:
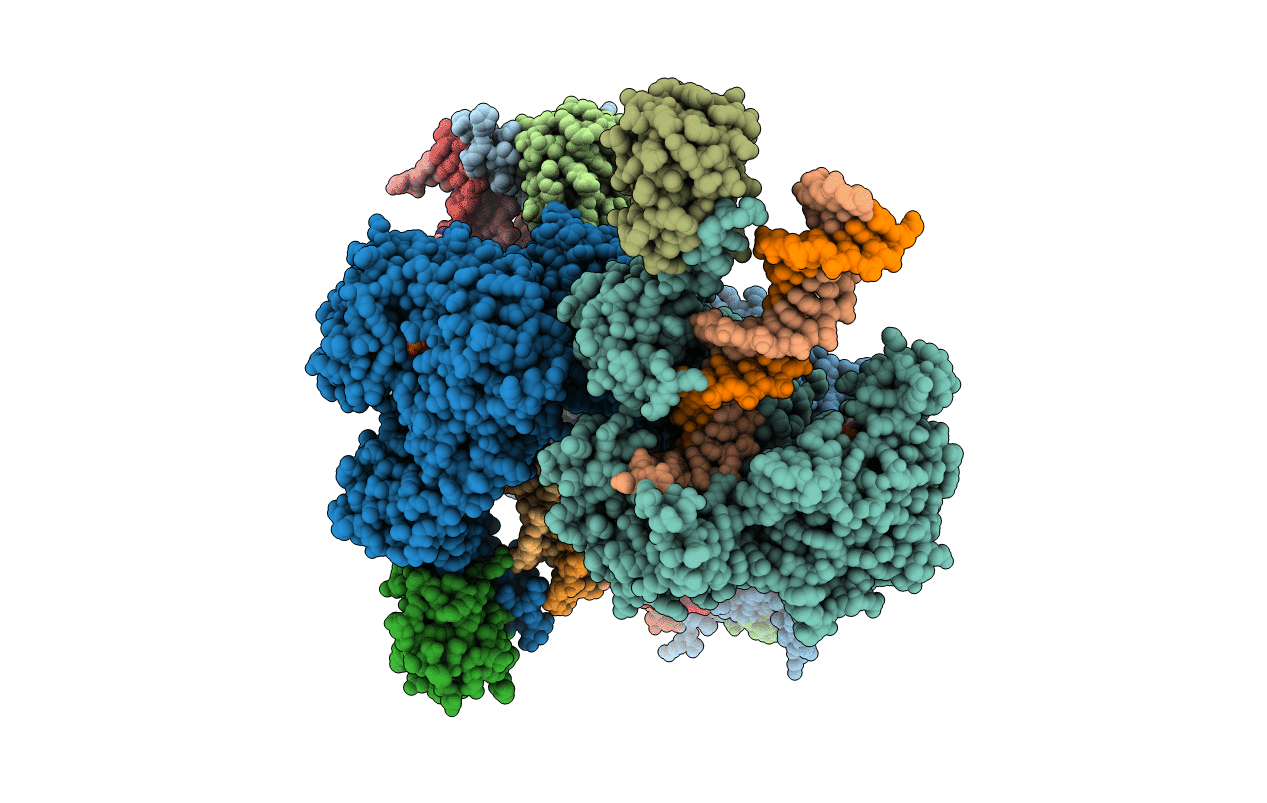
Structure of T7 DNA Polymerase Bound to a Primer/Template DNA and a Peptide that Mimics the C-terminal Tail of the Primase-Helicase
Biological Source:
Source Organism(s):
Enterobacteria phage T7 (Taxon ID: 10760)
Escherichia coli (Taxon ID: 562)
Escherichia coli (Taxon ID: 562)
Expression System(s):
Method Details:
Experimental Method:
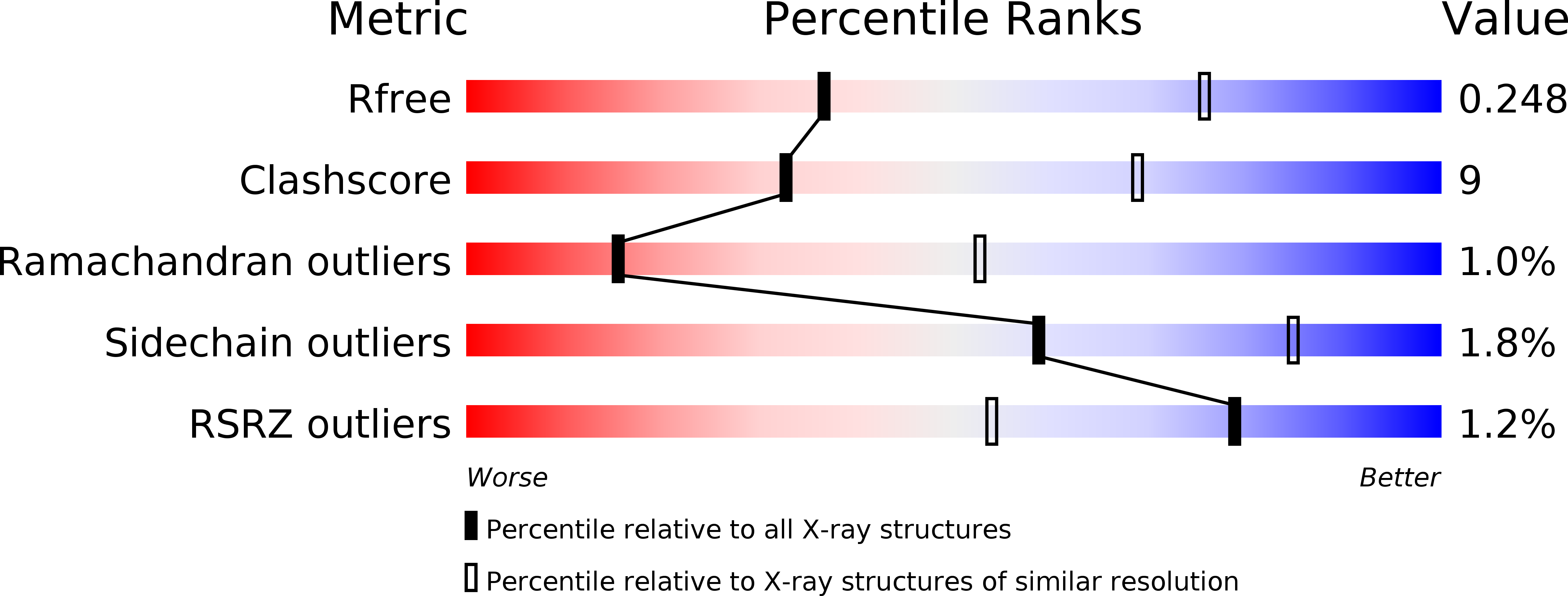
Resolution:
3.00 Å
R-Value Free:
0.24
R-Value Work:
0.21
R-Value Observed:
0.21
Space Group:
P 1