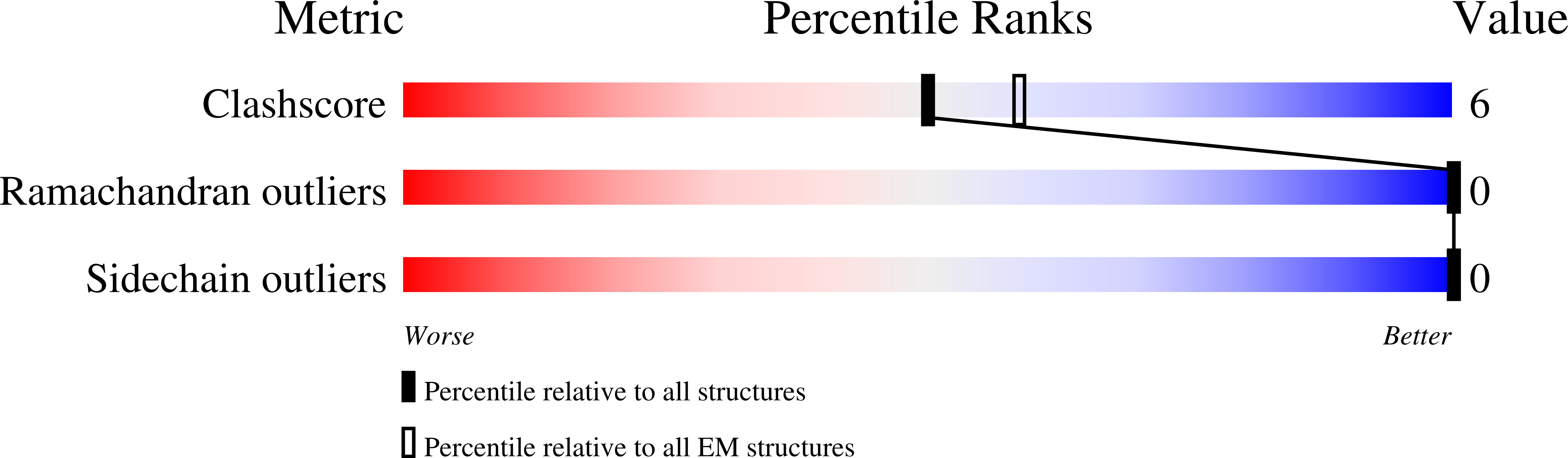
Deposition Date
2019-05-05
Release Date
2019-06-26
Last Version Date
2024-03-20
Entry Detail
PDB ID:
6OUT
Keywords:
Title:
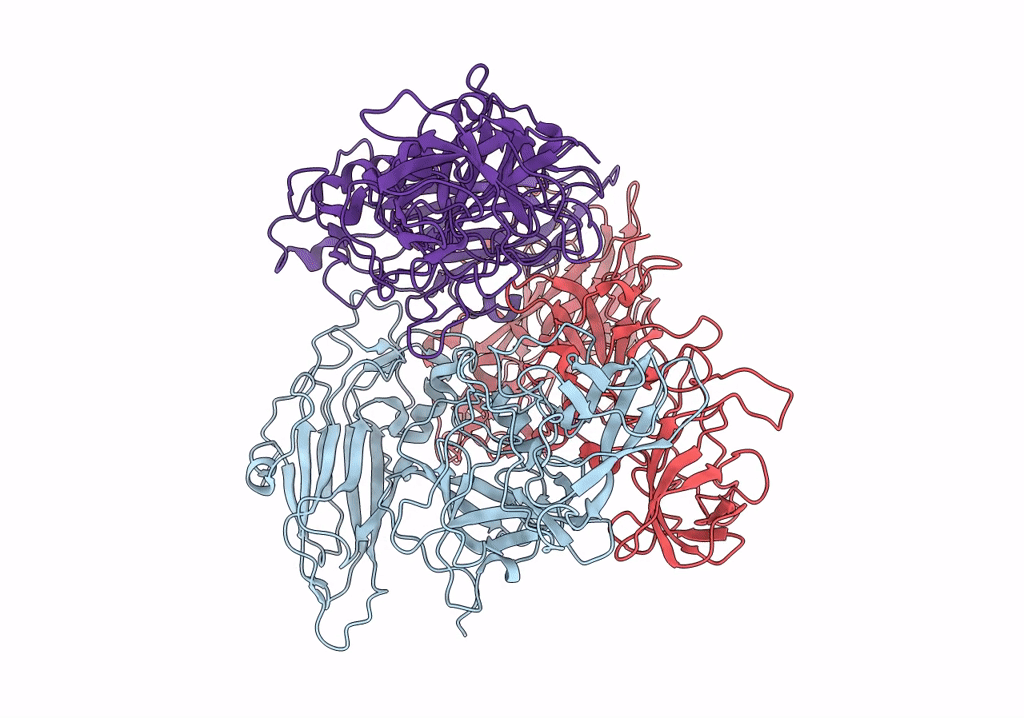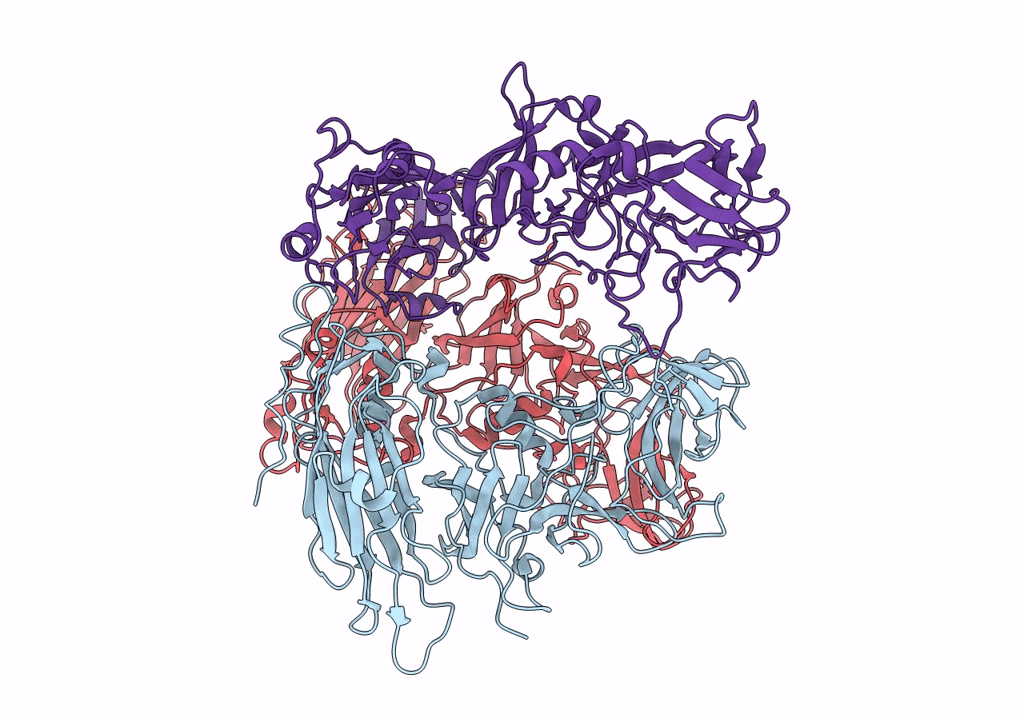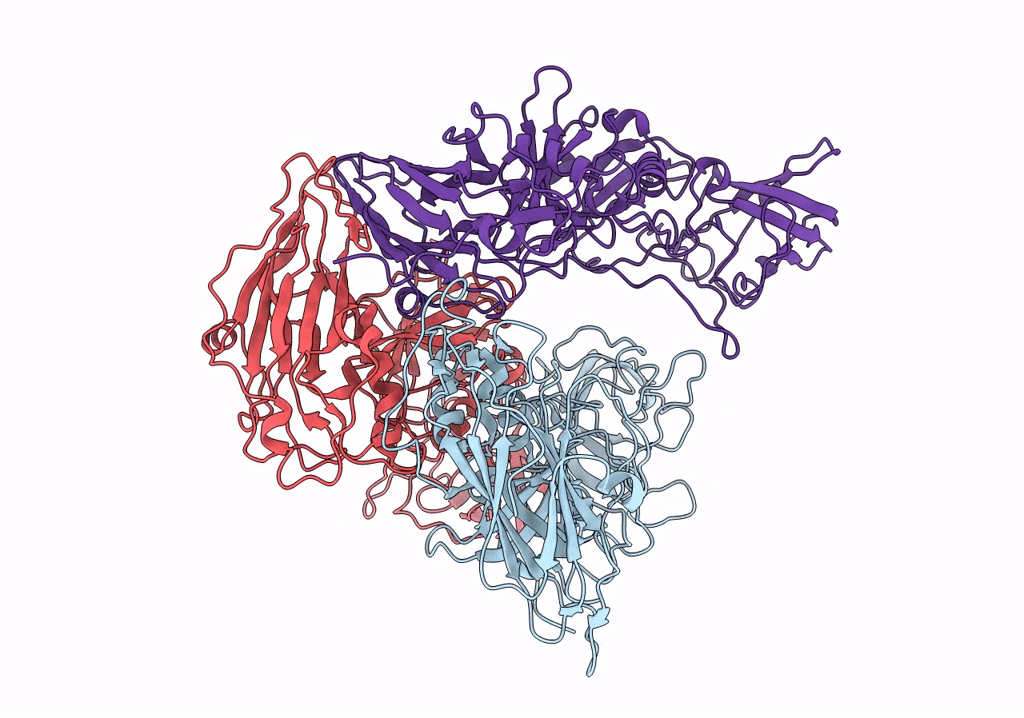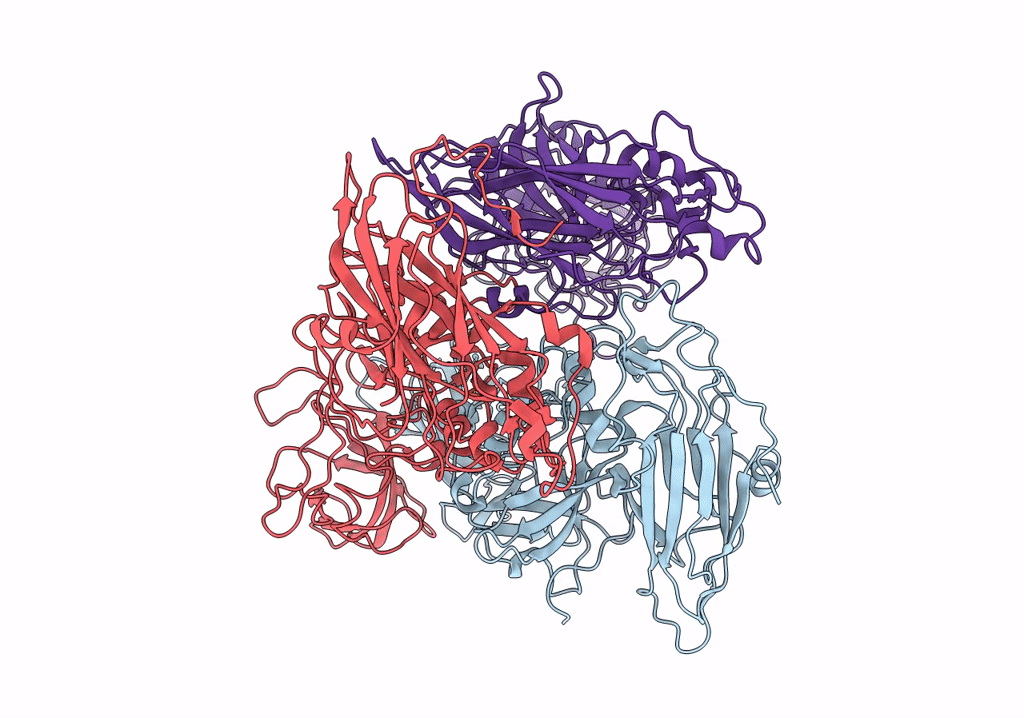
Asymmetric focused reconstruction of human norovirus GI.1 Norwalk strain VLP asymmetric unit in T=3 symmetry
Biological Source:
Source Organism(s):
Expression System(s):
Method Details:
Experimental Method:
Resolution:
2.60 Å
Aggregation State:
PARTICLE
Reconstruction Method:
SINGLE PARTICLE