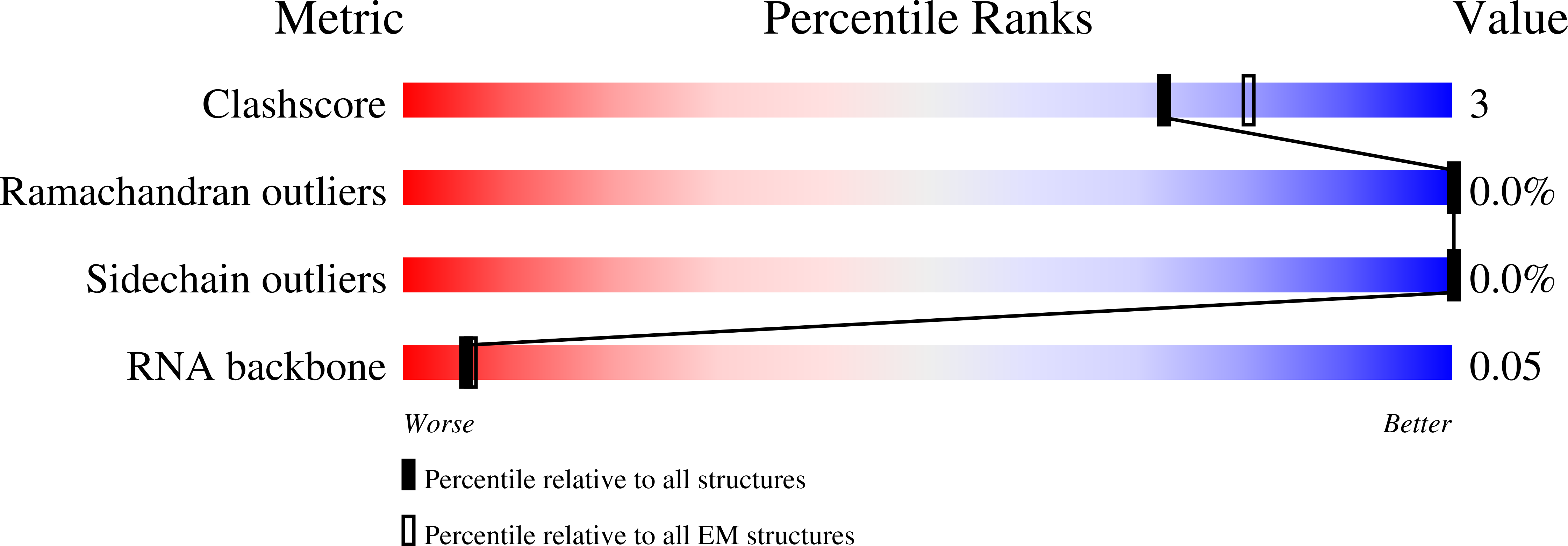
Deposition Date
2019-04-03
Release Date
2019-05-22
Last Version Date
2025-05-21
Entry Detail
PDB ID:
6OGY
Keywords:
Title:
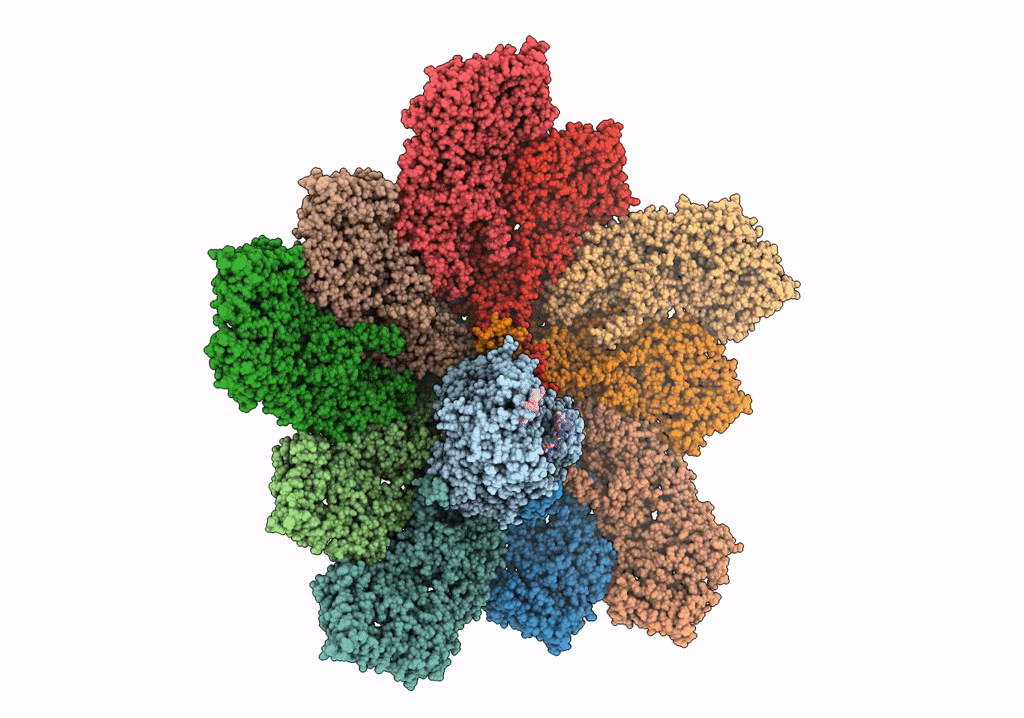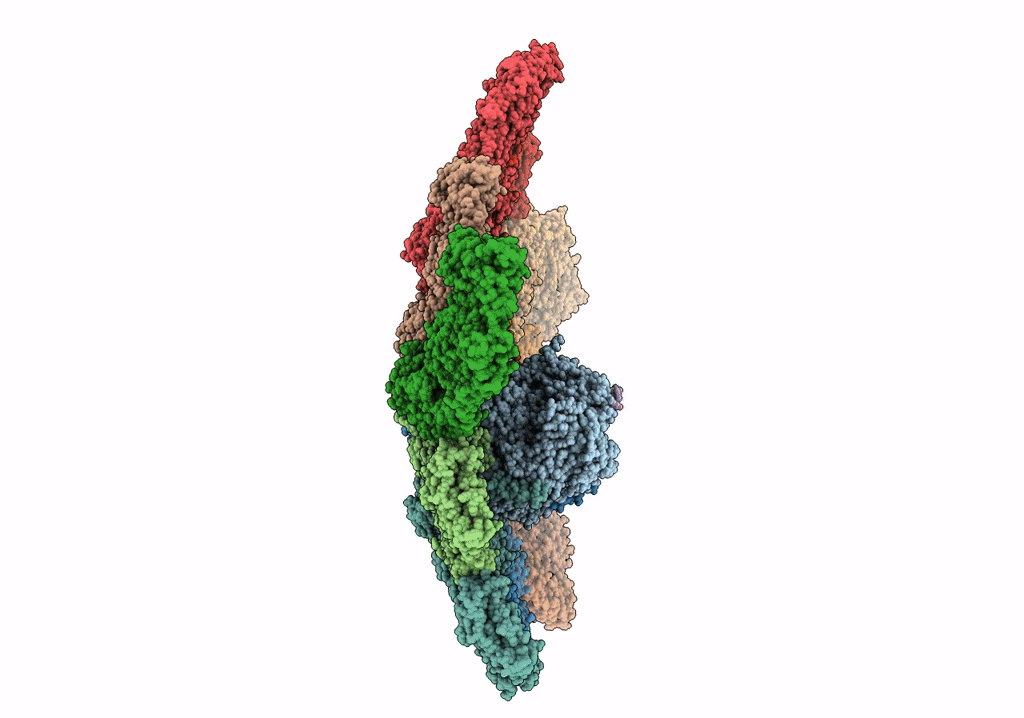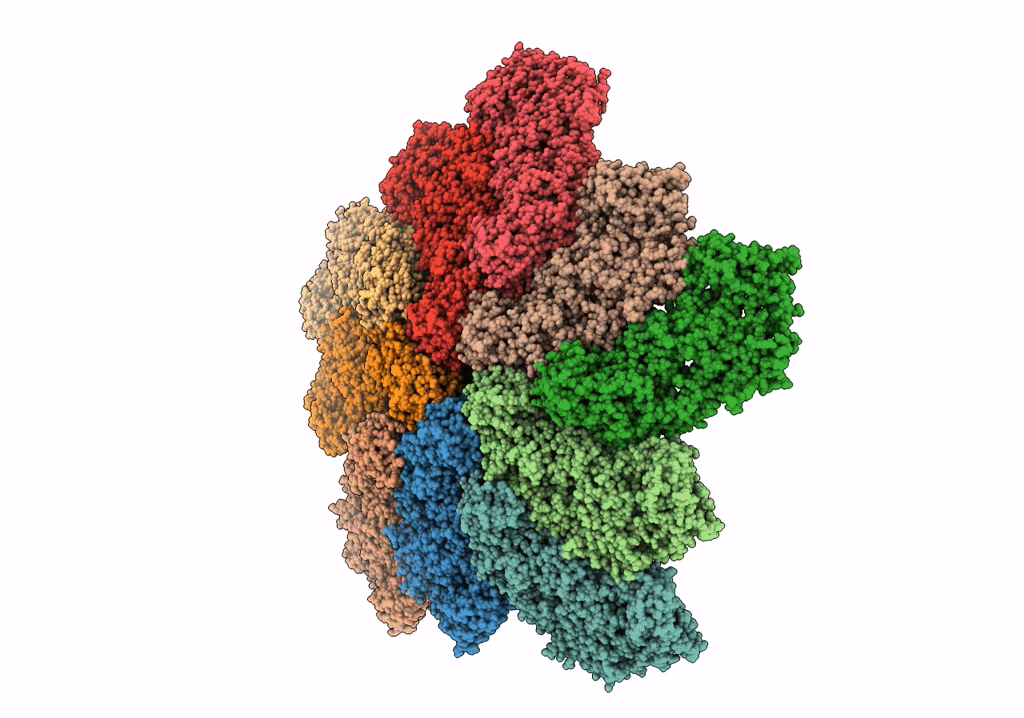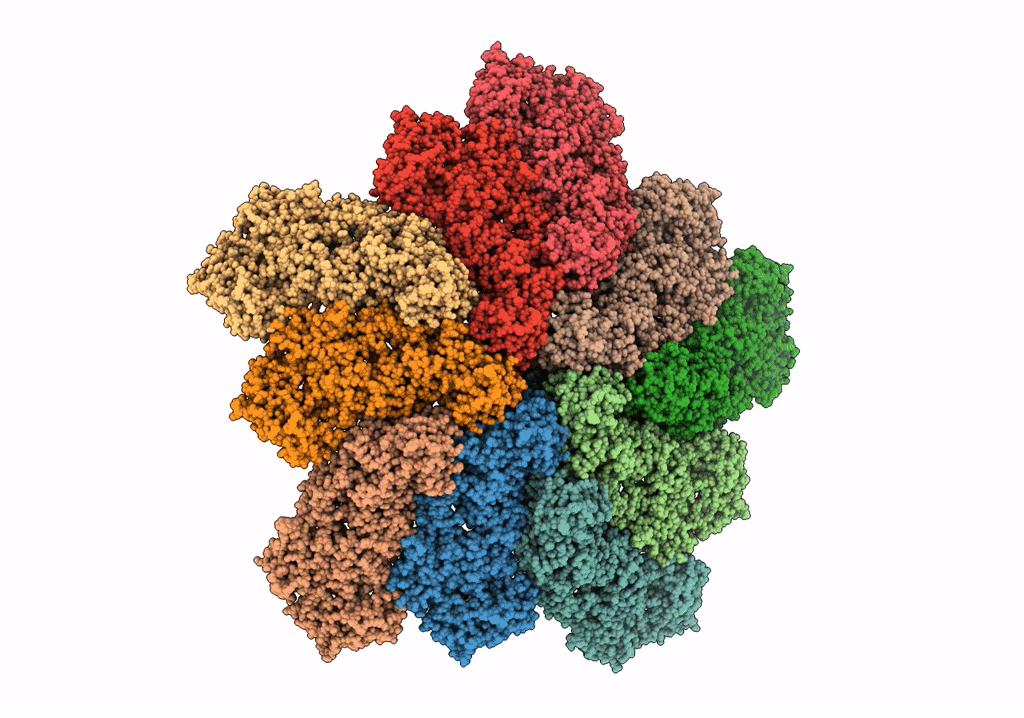
In situ structure of Rotavirus RNA-dependent RNA polymerase at duplex-open state
Biological Source:
Source Organism:
Rotavirus A (Taxon ID: 28875)
Method Details:
Experimental Method:
Resolution:
3.40 Å
Aggregation State:
PARTICLE
Reconstruction Method:
SINGLE PARTICLE