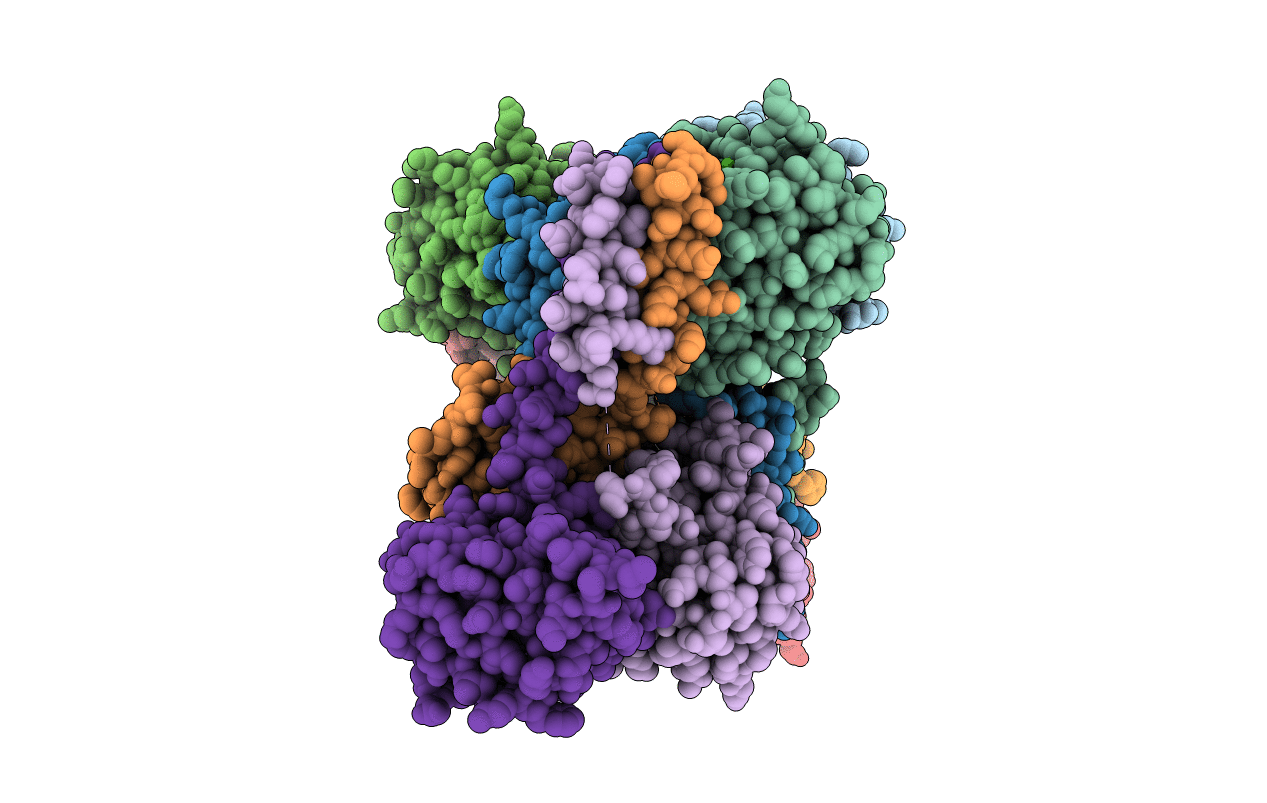
Deposition Date
2019-03-27
Release Date
2019-04-24
Last Version Date
2023-10-11
Entry Detail
PDB ID:
6OEC
Keywords:
Title:
Yeast Spc42 Trimeric Coiled-Coil Amino Acids 181-211 fused to PDB: 3H5I
Biological Source:
Source Organism(s):
Expression System(s):
Method Details:
Experimental Method:
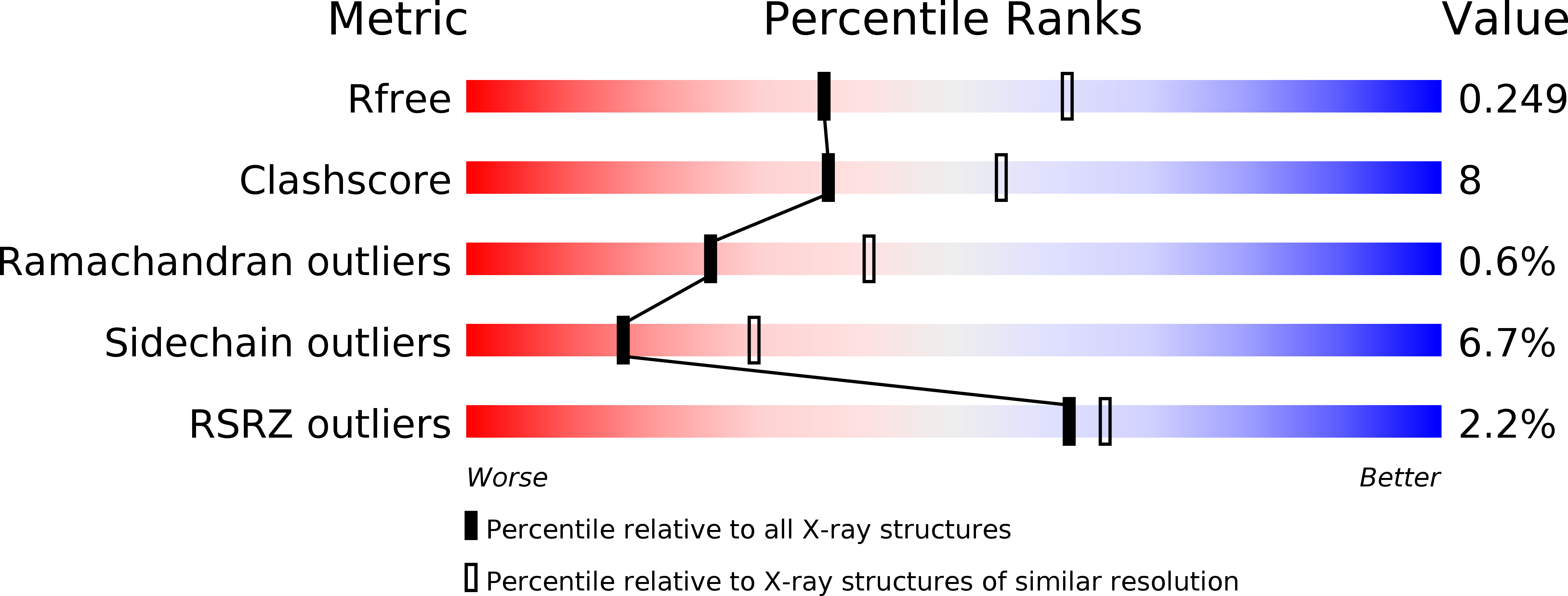
Resolution:
2.51 Å
R-Value Free:
0.24
R-Value Work:
0.19
R-Value Observed:
0.19
Space Group:
P 21 21 2