Deposition Date
2019-03-27
Release Date
2019-08-21
Last Version Date
2024-11-06
Entry Detail
PDB ID:
6OE8
Keywords:
Title:
The crystal structure of hyper-thermostable AgUricase mutant K12C/E286C
Biological Source:
Source Organism:
Arthrobacter globiformis (Taxon ID: 1665)
Host Organism:
Method Details:
Experimental Method:
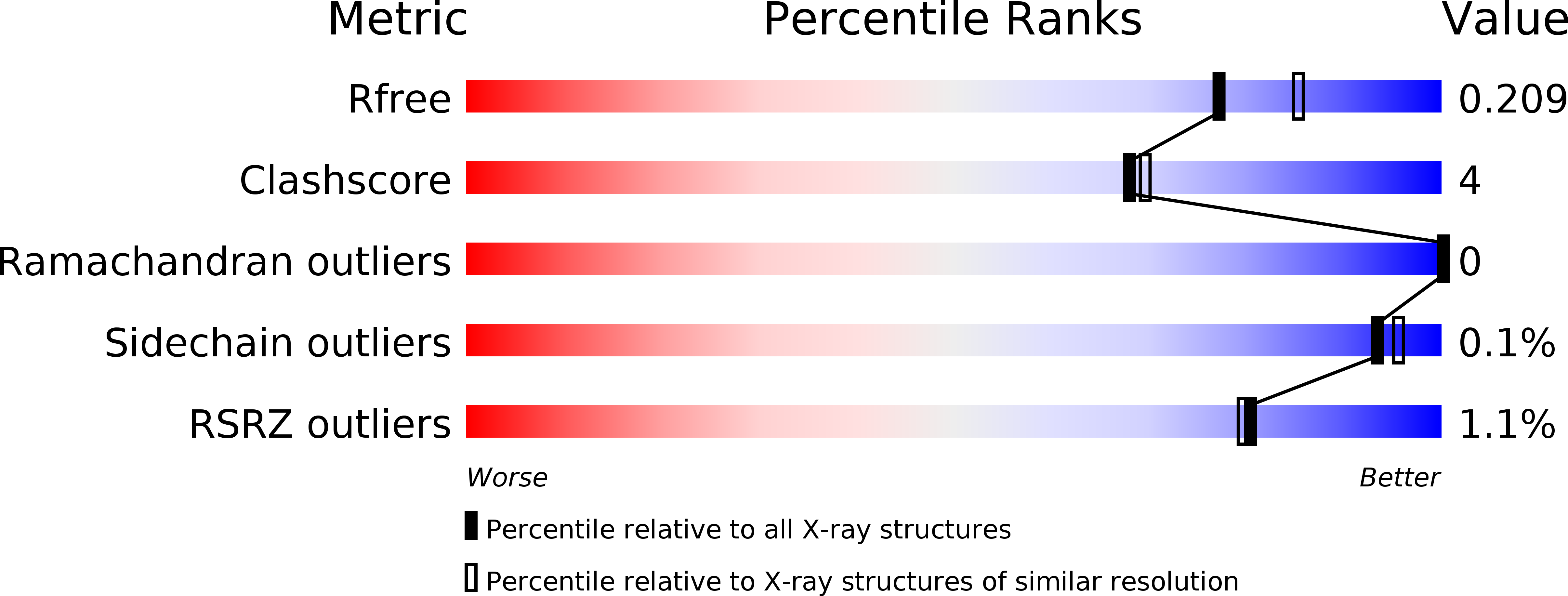
Resolution:
1.99 Å
R-Value Free:
0.20
R-Value Work:
0.18
R-Value Observed:
0.18
Space Group:
C 1 2 1