Deposition Date
2019-03-27
Release Date
2019-04-17
Last Version Date
2024-11-06
Entry Detail
PDB ID:
6OE5
Keywords:
Title:
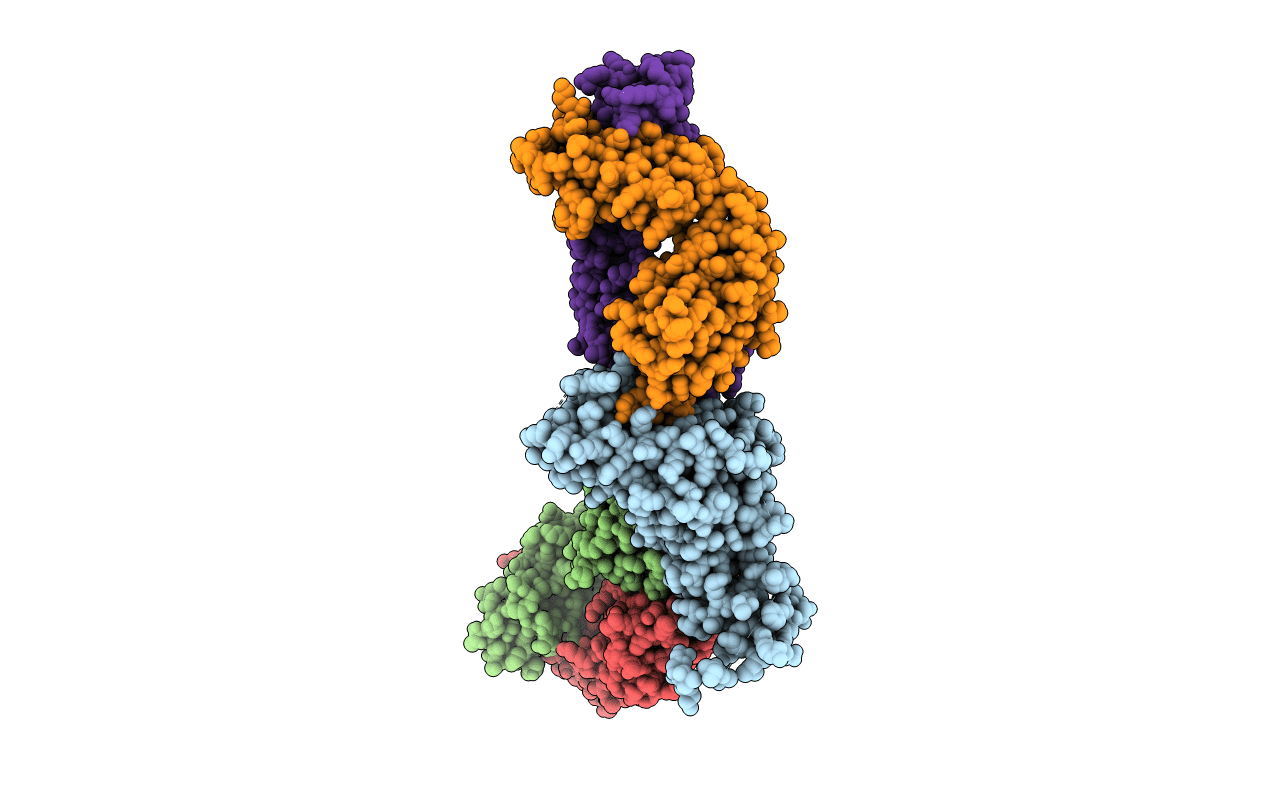
Splayed open prefusion RSV F captured by CR9501 and motavizumab Fabs
Biological Source:
Source Organism:
Human respiratory syncytial virus A (strain A2) (Taxon ID: 11259)
Enterobacteria phage T4 (Taxon ID: 10665)
Mus musculus (Taxon ID: 10090)
Homo sapiens (Taxon ID: 9606)
Enterobacteria phage T4 (Taxon ID: 10665)
Mus musculus (Taxon ID: 10090)
Homo sapiens (Taxon ID: 9606)
Host Organism:
Method Details:
Experimental Method:
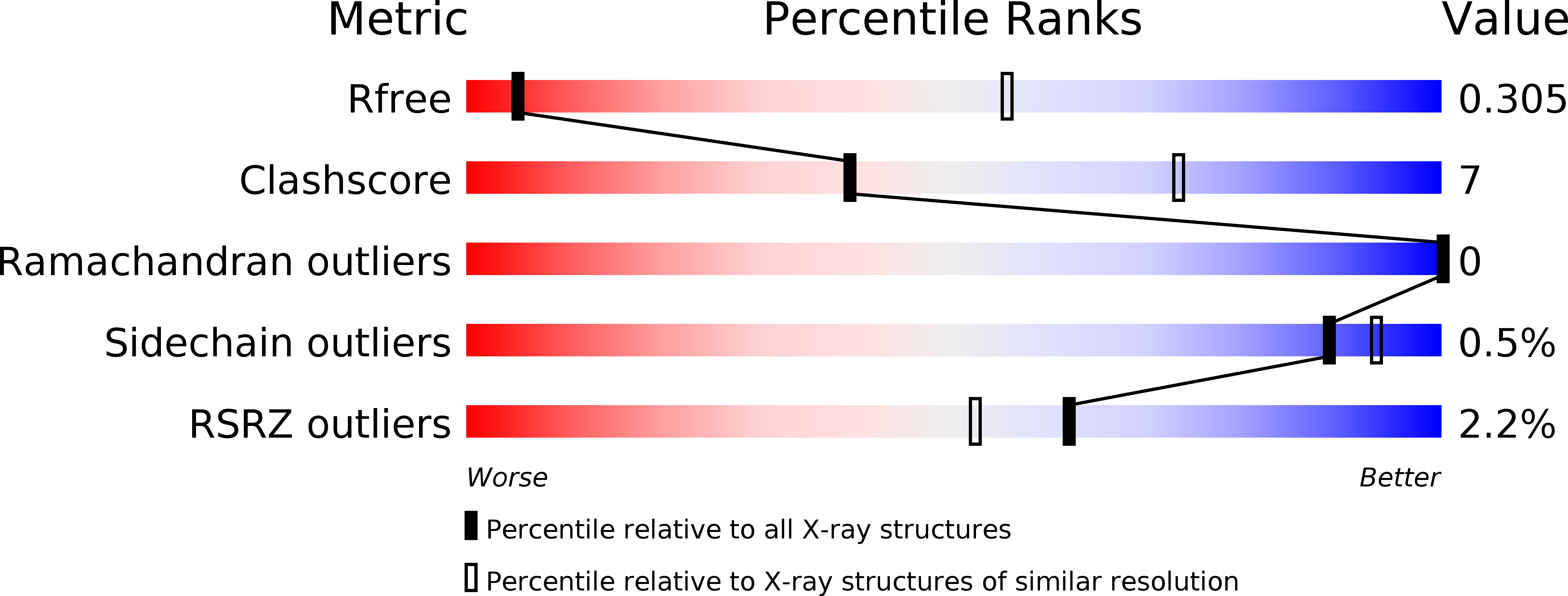
Resolution:
4.10 Å
R-Value Free:
0.30
R-Value Work:
0.26
R-Value Observed:
0.26
Space Group:
P 61