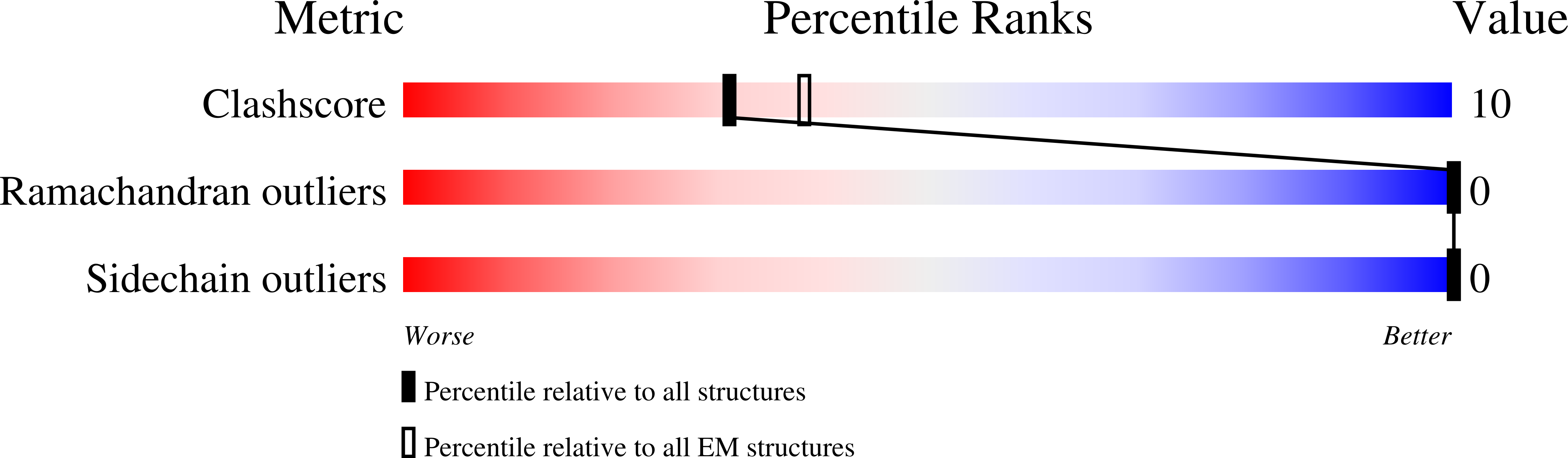
Deposition Date
2019-03-26
Release Date
2019-06-19
Last Version Date
2024-03-13
Entry Detail
PDB ID:
6OD7
Keywords:
Title:
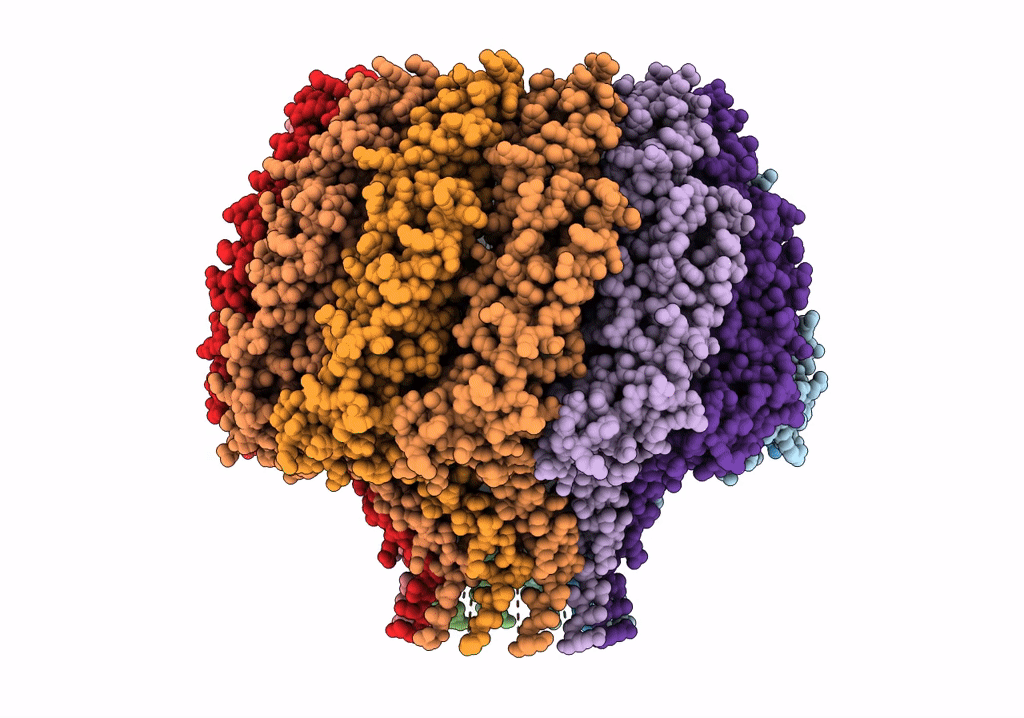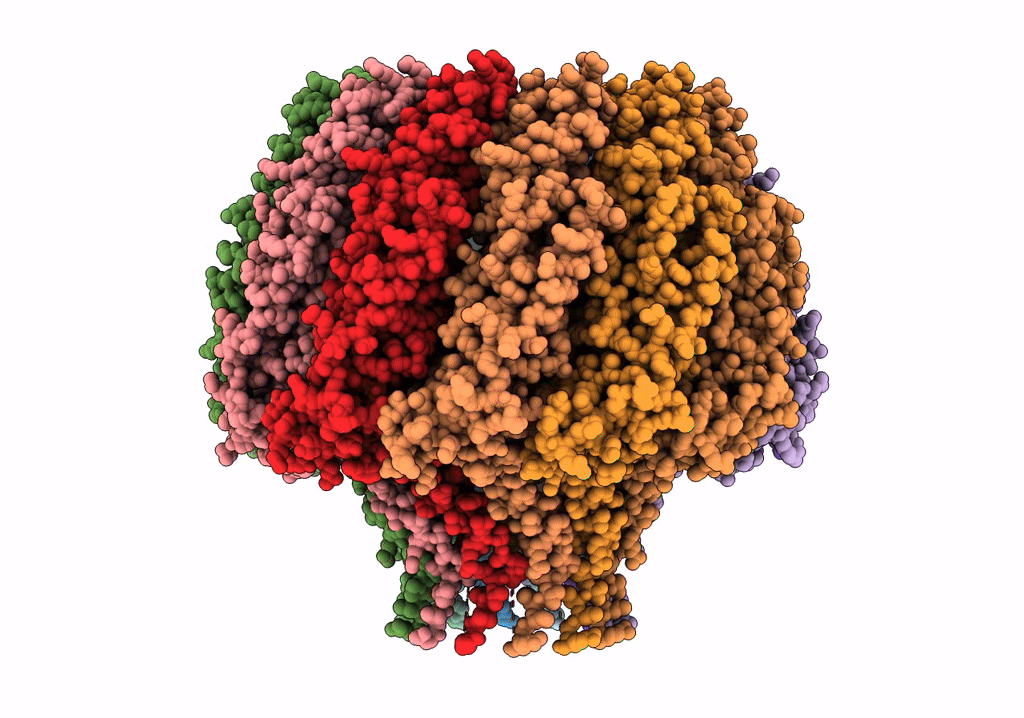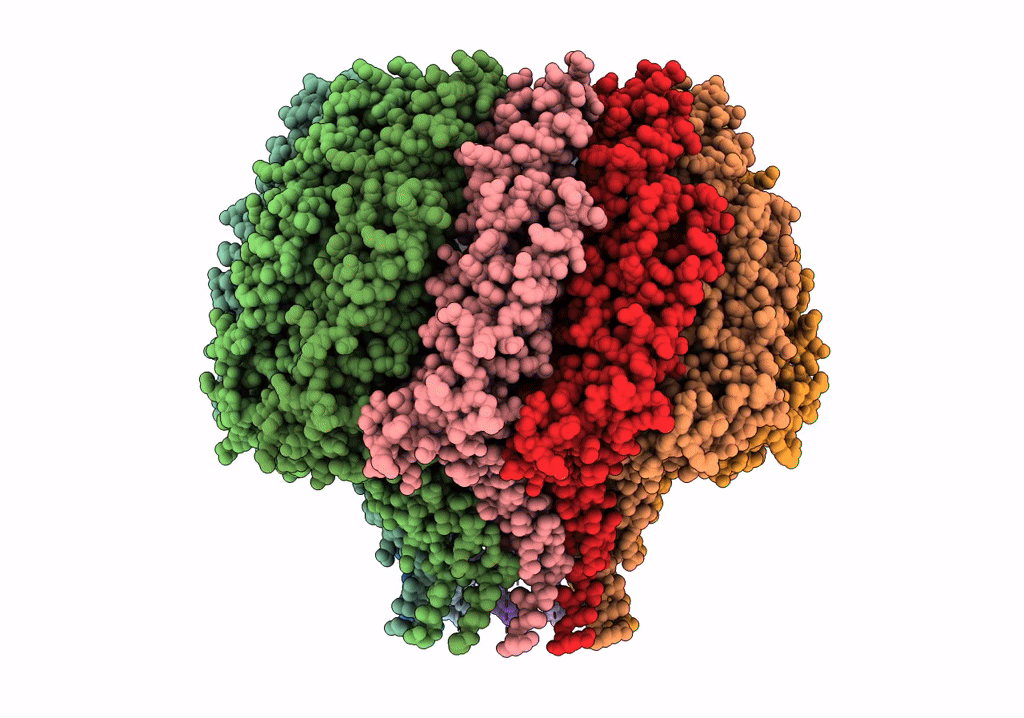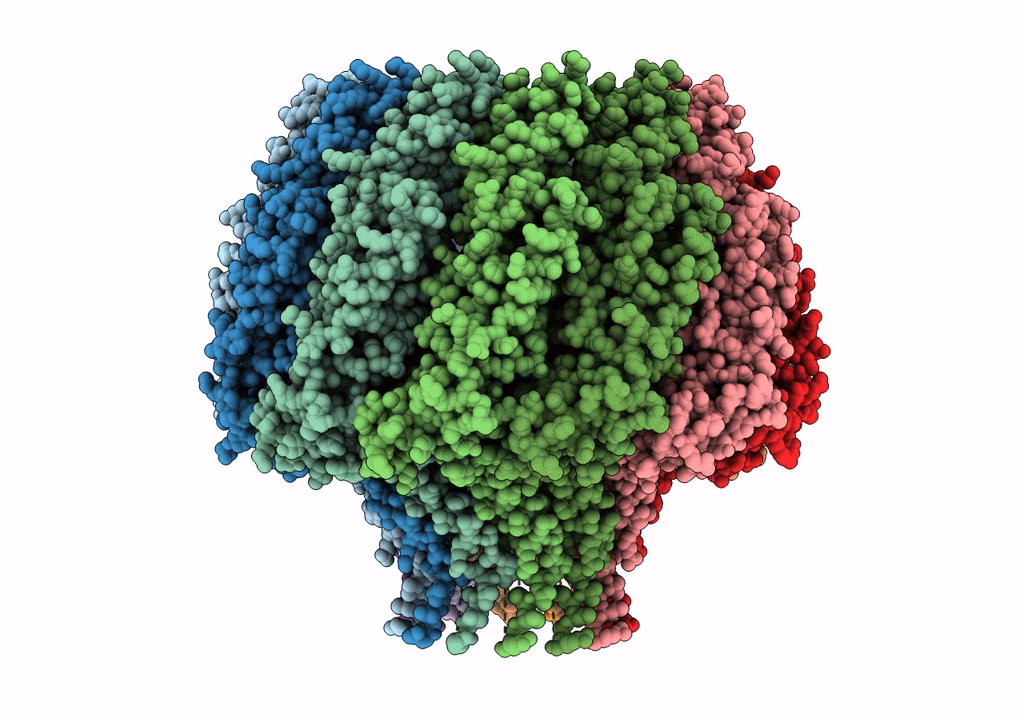
Herpes simplex virus type 1 (HSV-1) pUL6 portal protein, dodecameric complex
Biological Source:
Source Organism(s):
Human herpesvirus 1 strain KOS (Taxon ID: 10306)
Method Details:
Experimental Method:
Resolution:
5.60 Å
Aggregation State:
PARTICLE
Reconstruction Method:
SINGLE PARTICLE