Deposition Date
2019-03-08
Release Date
2019-06-19
Last Version Date
2023-10-11
Entry Detail
PDB ID:
6O7Q
Keywords:
Title:
Nitrogenase MoFeP mutant S188A from Azotobacter vinelandii in the dithionite reduced state after redox cycling
Biological Source:
Source Organism:
Azotobacter vinelandii (Taxon ID: 354)
Method Details:
Experimental Method:
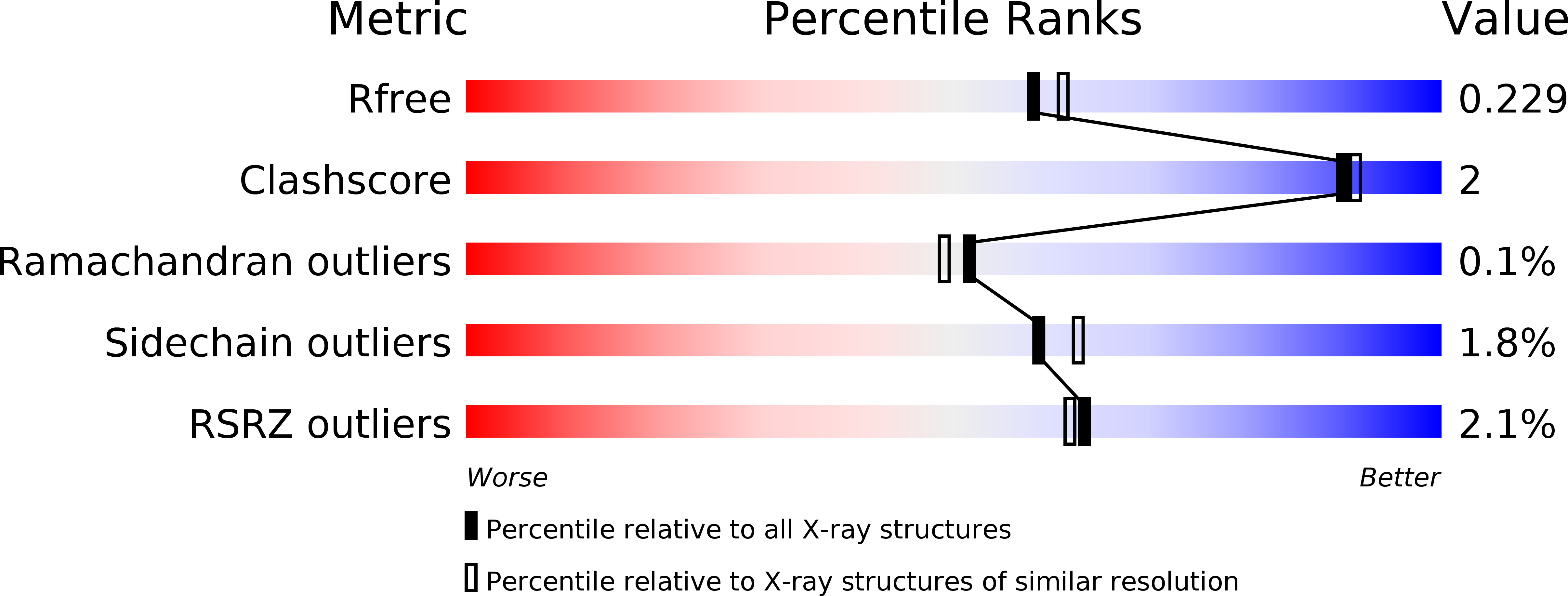
Resolution:
2.00 Å
R-Value Free:
0.22
R-Value Work:
0.18
R-Value Observed:
0.19
Space Group:
P 1 21 1