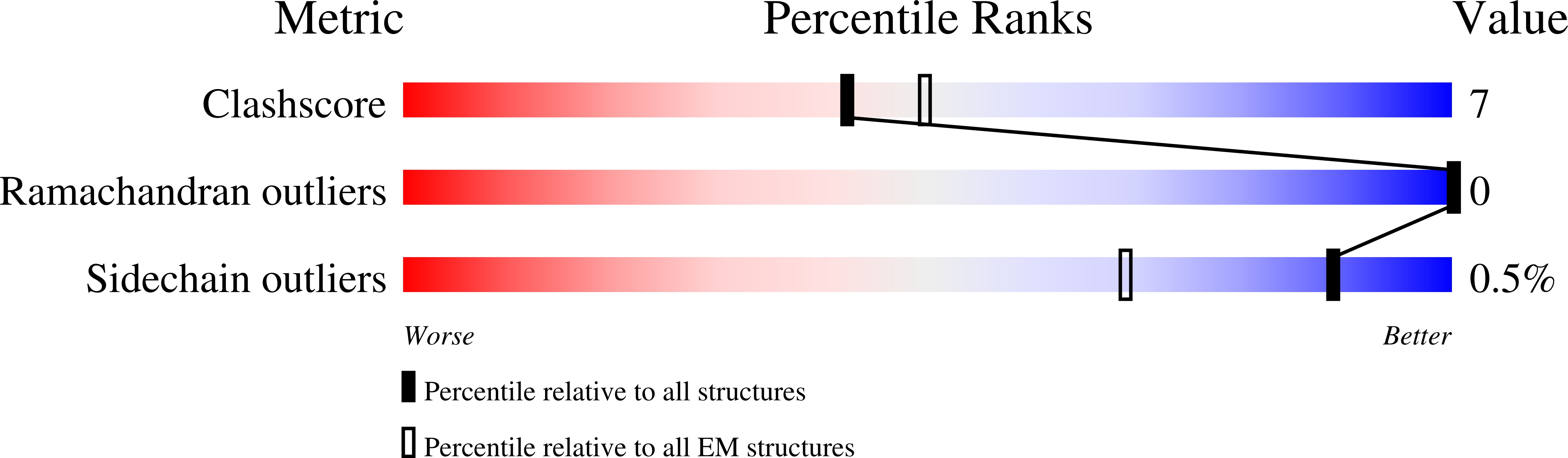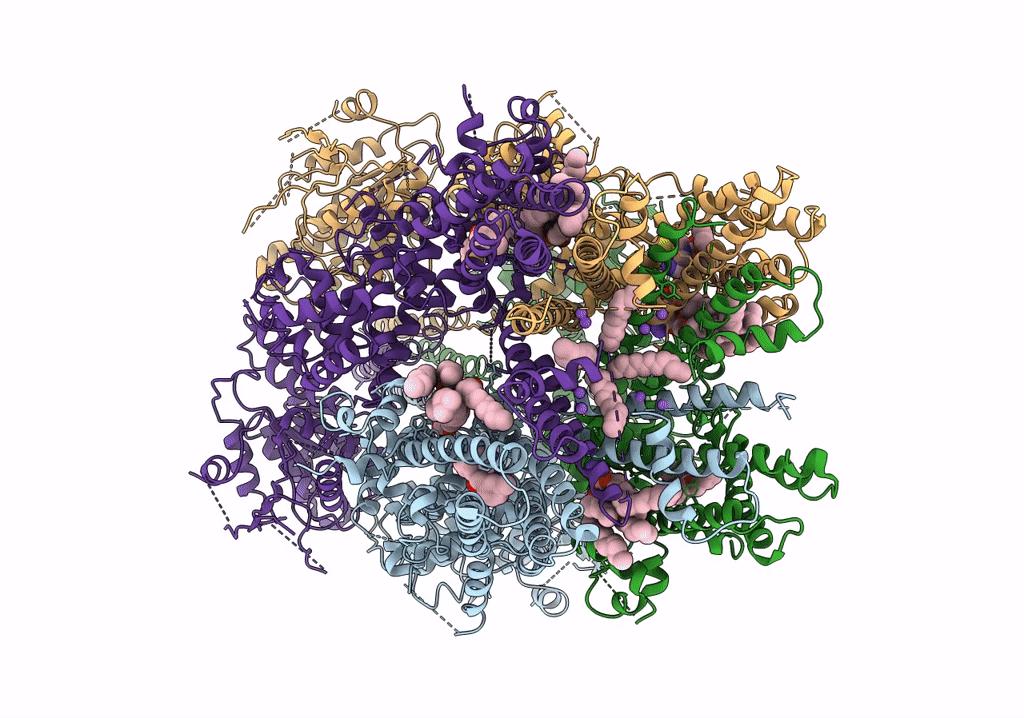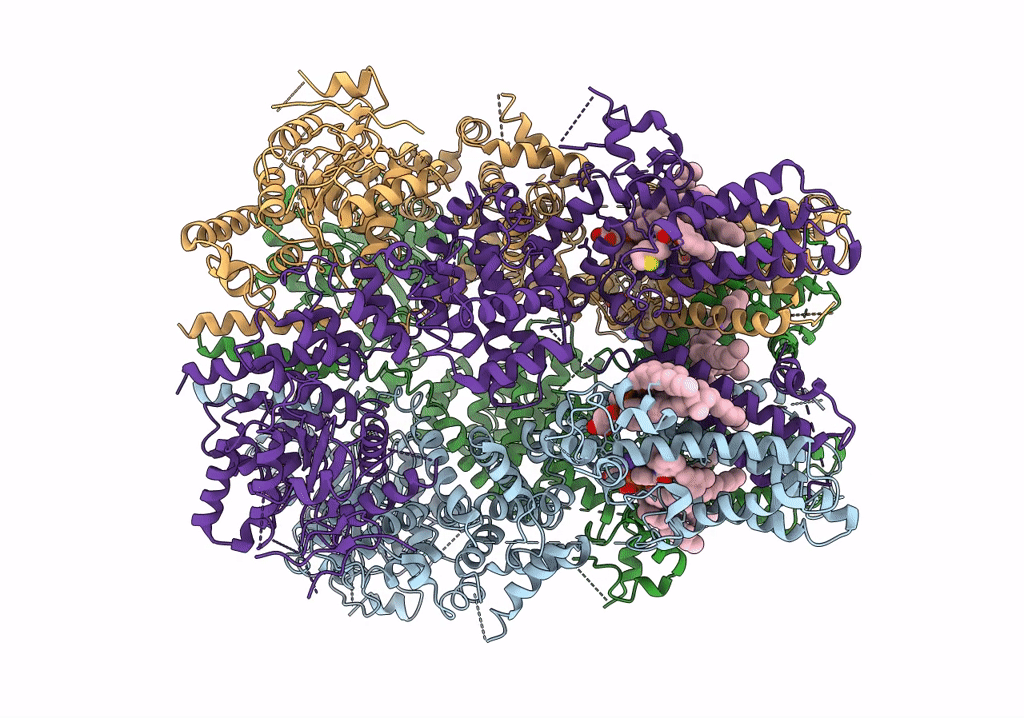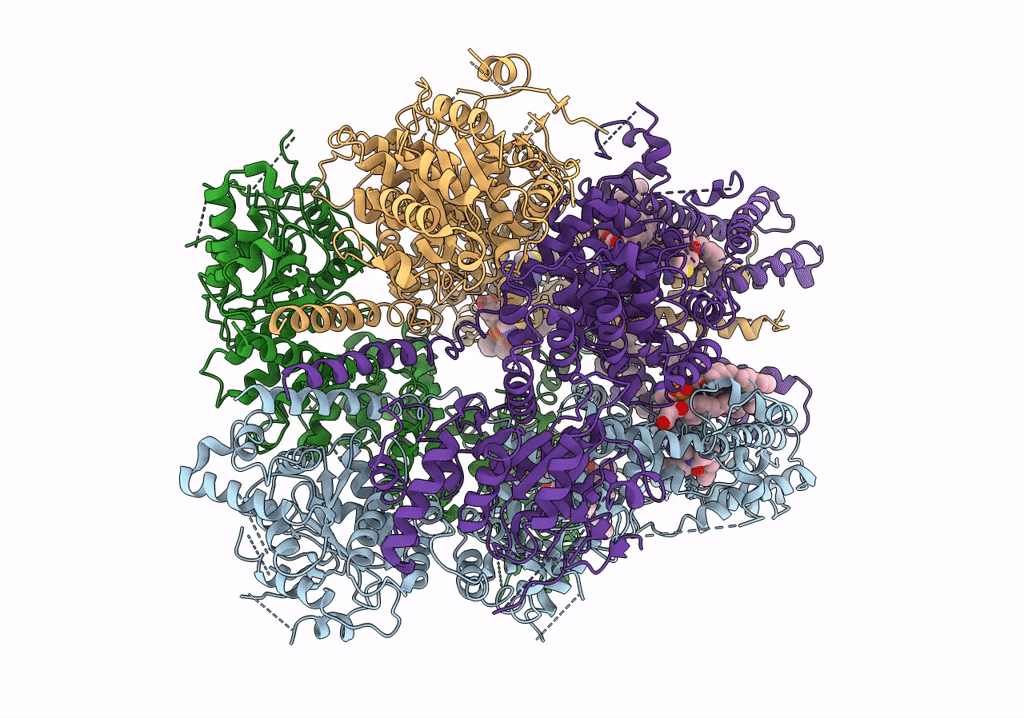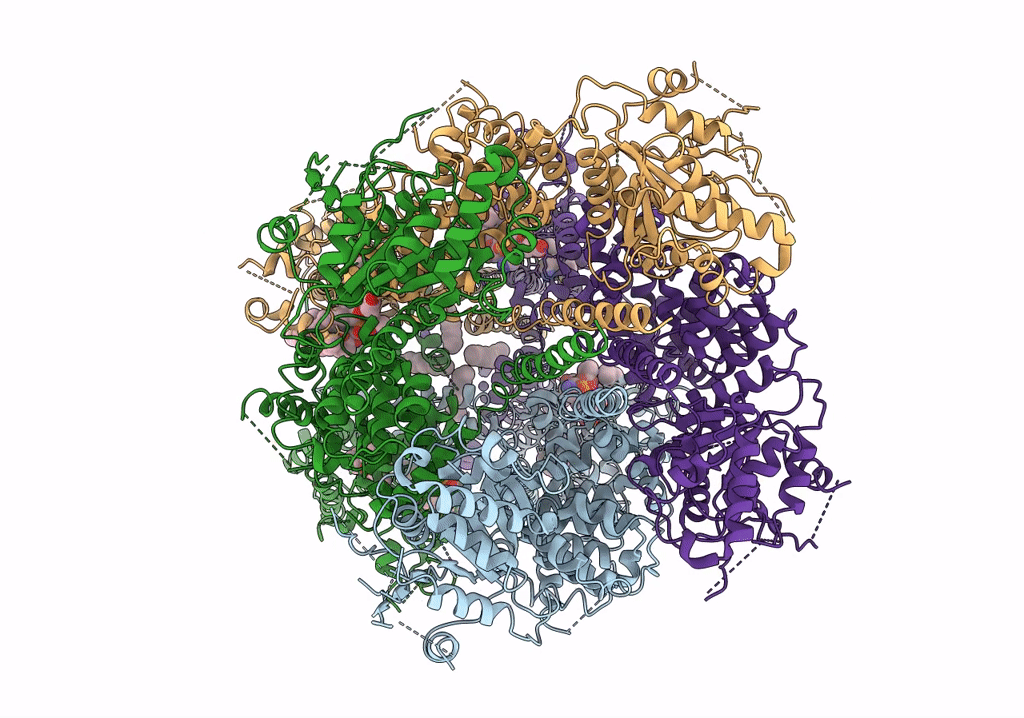
Deposition Date
2019-03-07
Release Date
2019-09-18
Last Version Date
2024-03-20
Entry Detail
PDB ID:
6O6R
Keywords:
Title:
Structure of the TRPM8 cold receptor by single particle electron cryo-microscopy, AMTB-bound state
Biological Source:
Source Organism(s):
Parus major (Taxon ID: 9157)
Expression System(s):
Method Details:
Experimental Method:
Resolution:
3.20 Å
Aggregation State:
PARTICLE
Reconstruction Method:
SINGLE PARTICLE