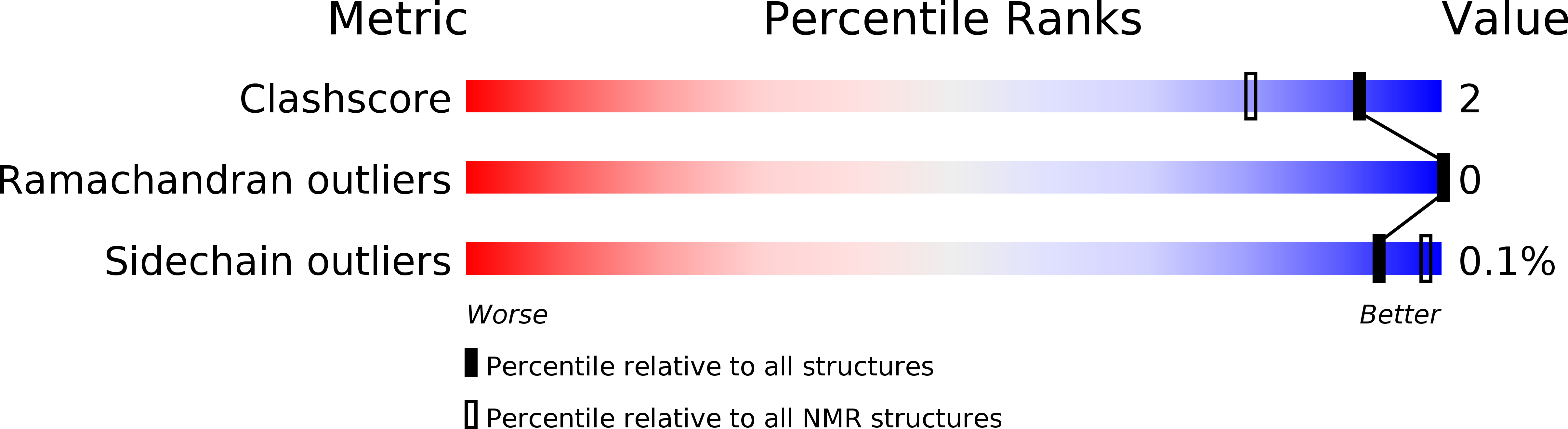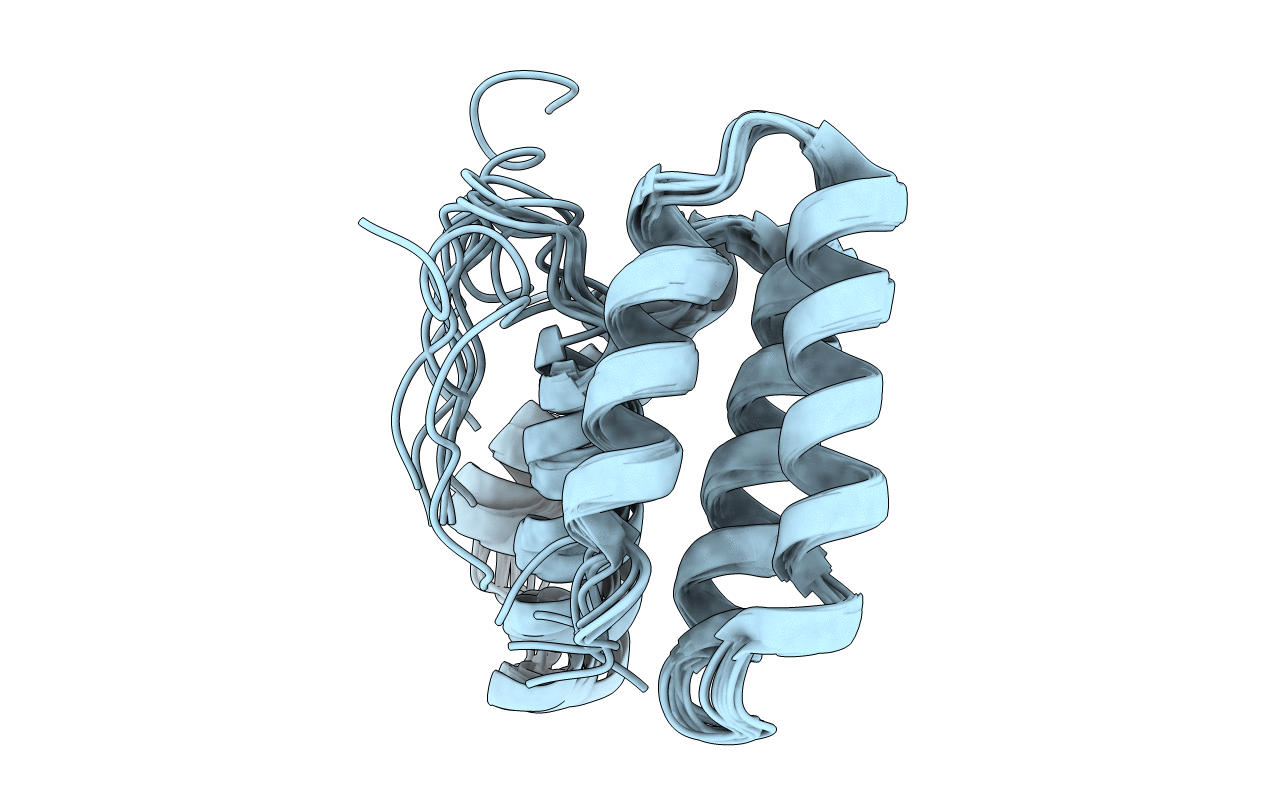
Deposition Date
2019-03-06
Release Date
2019-07-24
Last Version Date
2024-05-01
Entry Detail
PDB ID:
6O6I
Keywords:
Title:
Endoplasmic reticulum protein 29 (ERp29) C-terminal domain: Structure Determination from Backbone Amide Pseudocontact Shifts Generated by Double-histidine Cobalt Tags
Biological Source:
Source Organism:
Rattus norvegicus (Taxon ID: 10116)
Host Organism:
Method Details:
Experimental Method:
Conformers Calculated:
20000
Conformers Submitted:
10
Selection Criteria:
target function