Deposition Date
2019-02-22
Release Date
2019-07-31
Last Version Date
2024-05-01
Entry Detail
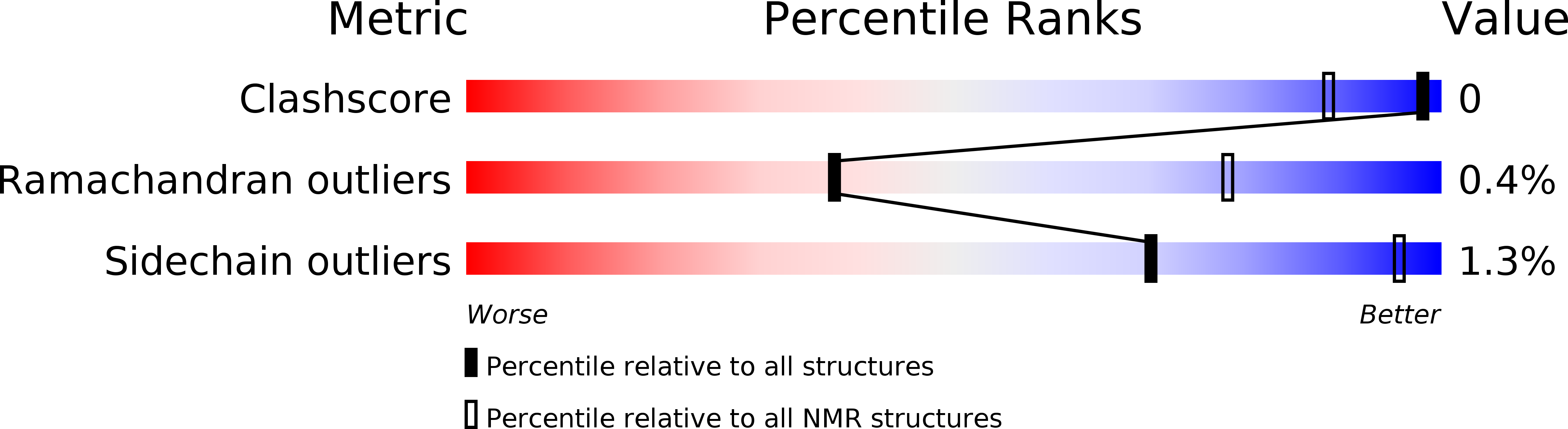
PDB ID:
6O22
Keywords:
Title:
Structure of Asf1-H3:H4-Rtt109-Vps75 histone chaperone-lysine acetyltransferase complex with the histone substrate.
Biological Source:
Source Organism(s):
Saccharomyces cerevisiae (strain ATCC 204508 / S288c) (Taxon ID: 559292)
Xenopus laevis (Taxon ID: 8355)
Xenopus laevis (Taxon ID: 8355)
Expression System(s):
Method Details:
Experimental Method:
Conformers Calculated:
150
Conformers Submitted:
1
Selection Criteria:
structures with the least restraint violations