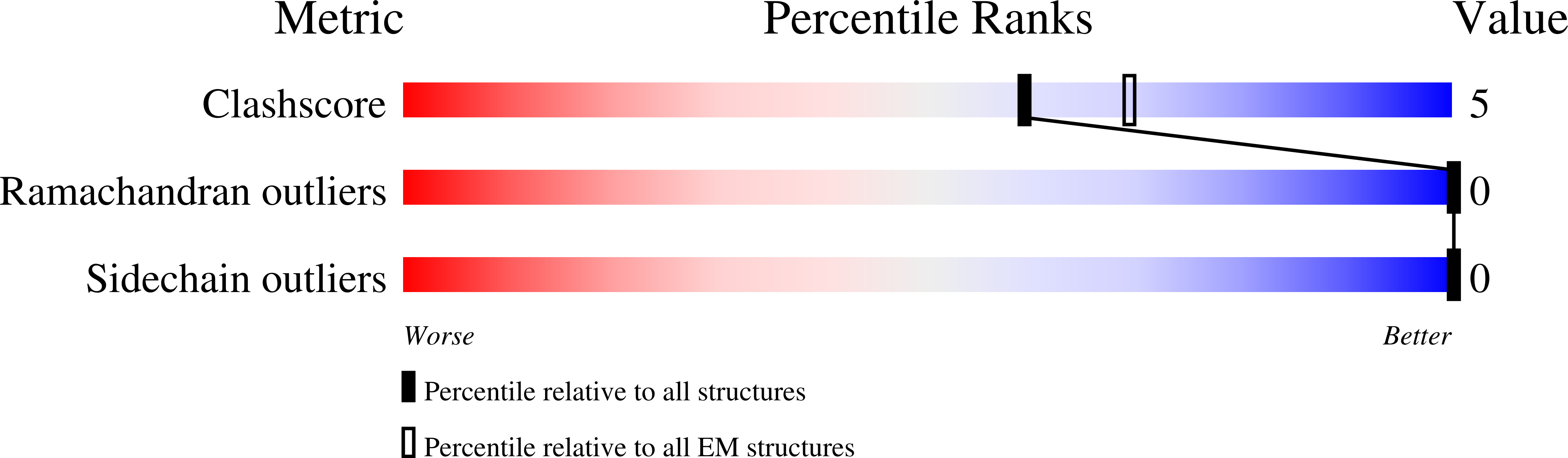
Deposition Date
2019-01-24
Release Date
2019-04-03
Last Version Date
2024-03-20
Entry Detail
PDB ID:
6NSK
Keywords:
Title:
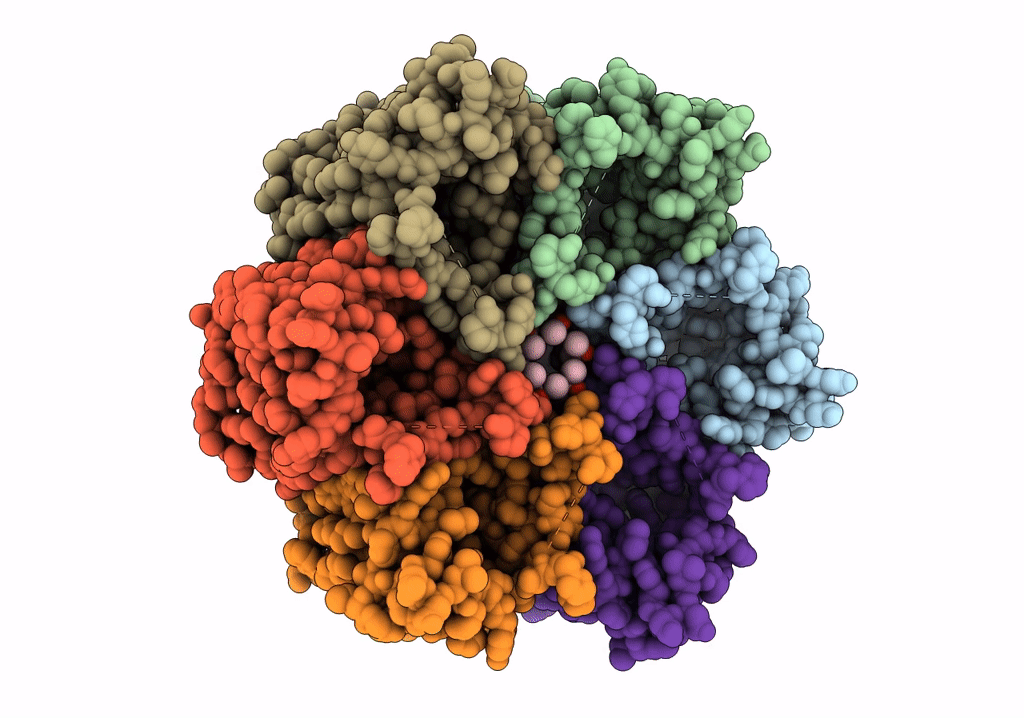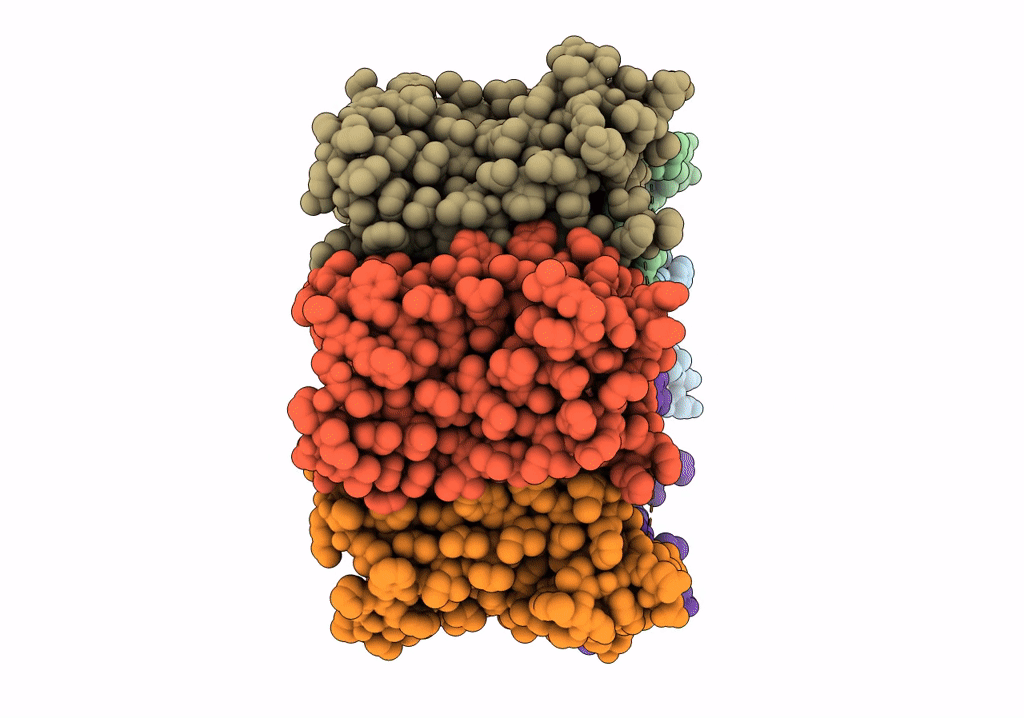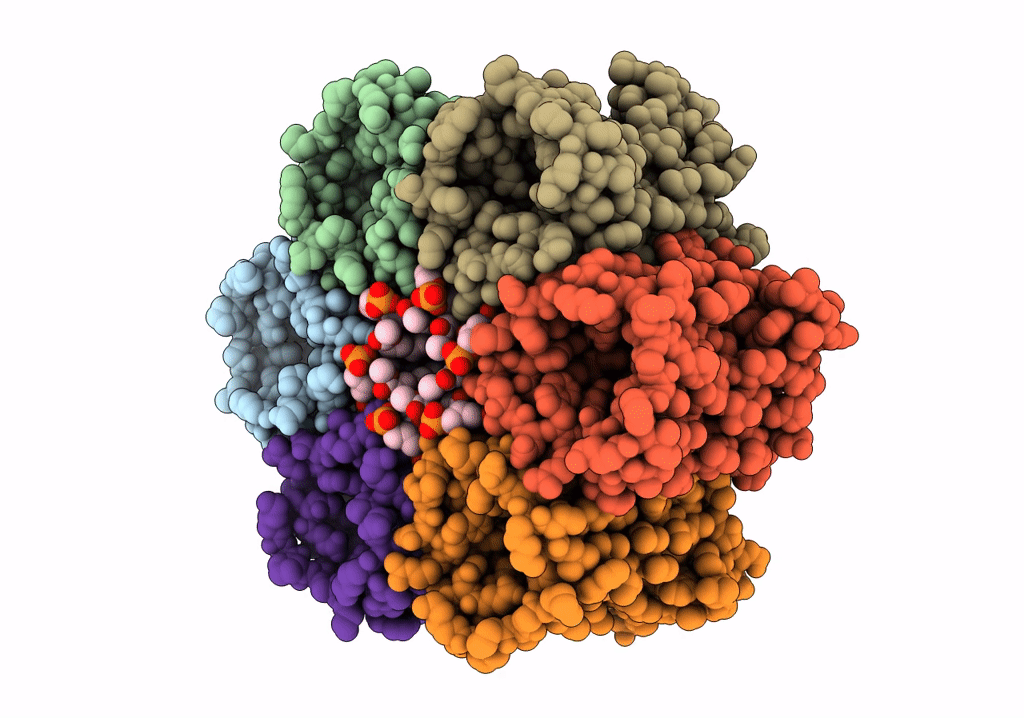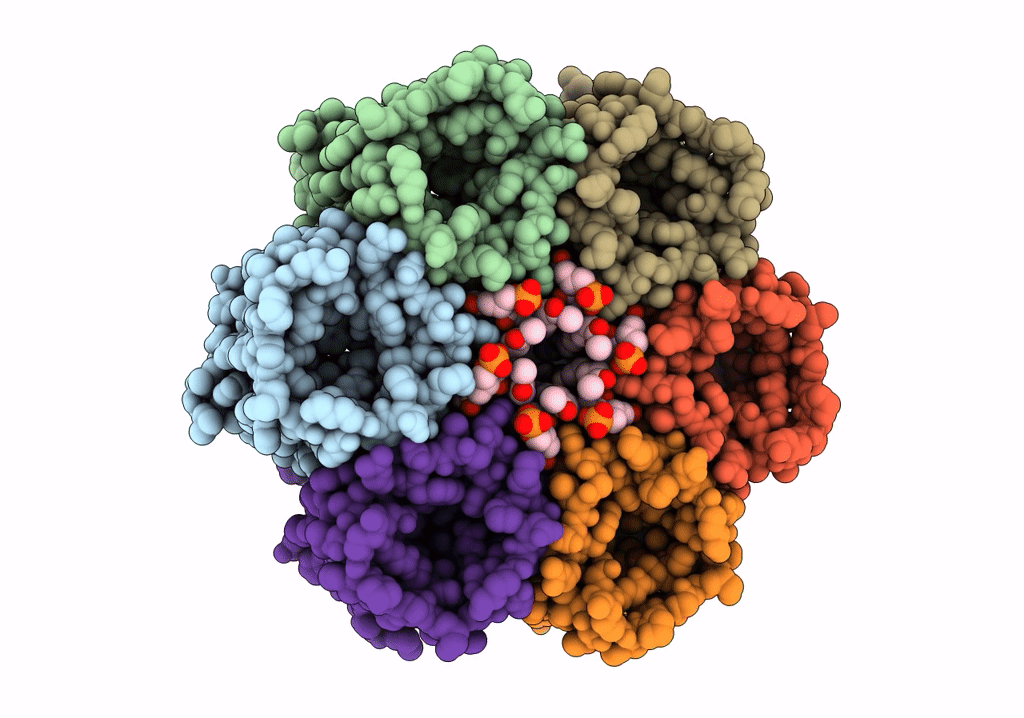
CryoEM structure of Helicobacter pylori urea channel in open state.
Biological Source:
Source Organism(s):
Helicobacter pylori (strain J99 / ATCC 700824) (Taxon ID: 85963)
Expression System(s):
Method Details:
Experimental Method:
Resolution:
2.70 Å
Aggregation State:
2D ARRAY
Reconstruction Method:
SINGLE PARTICLE