Deposition Date
2019-01-22
Release Date
2020-01-22
Last Version Date
2023-10-11
Entry Detail
PDB ID:
6NR0
Keywords:
Title:
SIRT2(56-356) with covalent intermediate between mechanism-based inhibitor Glucose-TM-1beta and 1'-SH ADP-ribose
Biological Source:
Source Organism:
Homo sapiens (Taxon ID: 9606)
Host Organism:
Method Details:
Experimental Method:
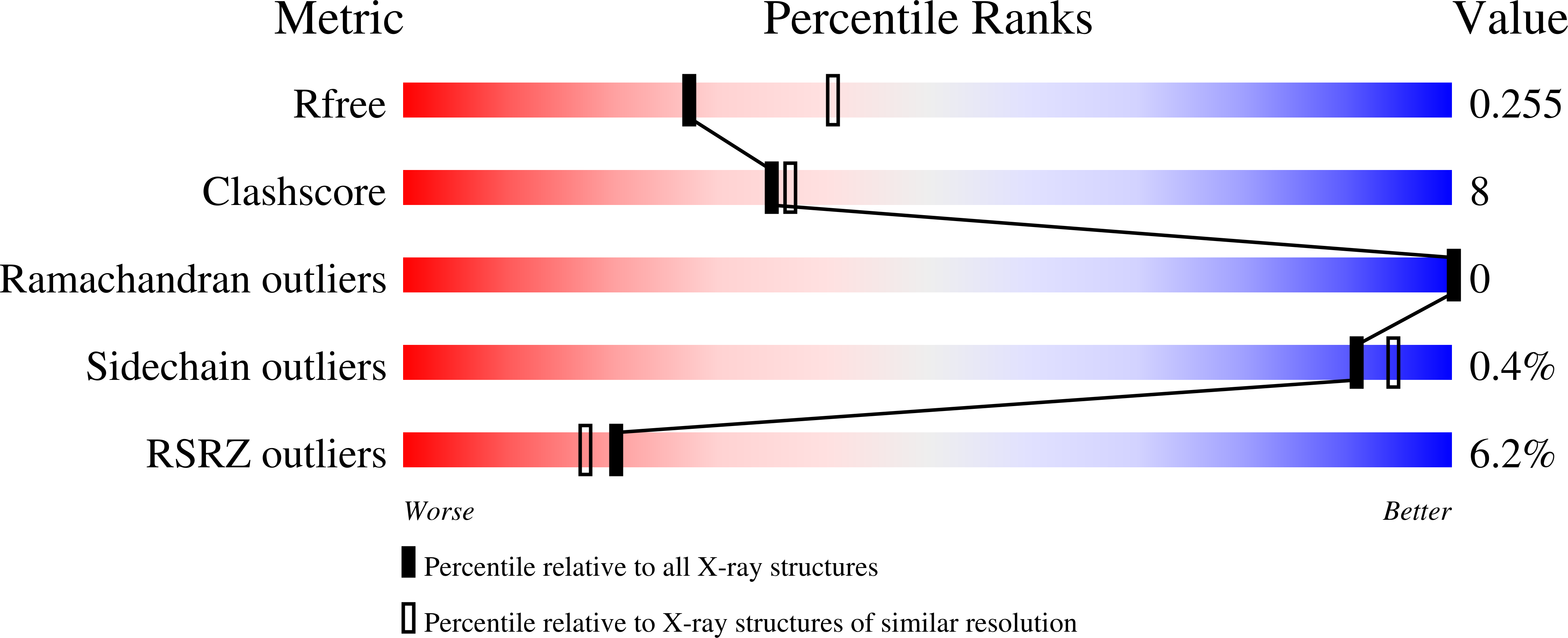
Resolution:
2.45 Å
R-Value Free:
0.25
R-Value Work:
0.22
R-Value Observed:
0.22
Space Group:
I 1 2 1