Deposition Date
2019-01-15
Release Date
2019-07-17
Last Version Date
2024-11-20
Entry Detail
PDB ID:
6NO4
Keywords:
Title:
ADP bound to L227bF mutant ATP-grasp fold of Blastocystis hominis succinyl-CoA synthetase
Biological Source:
Source Organism(s):
Expression System(s):
Method Details:
Experimental Method:
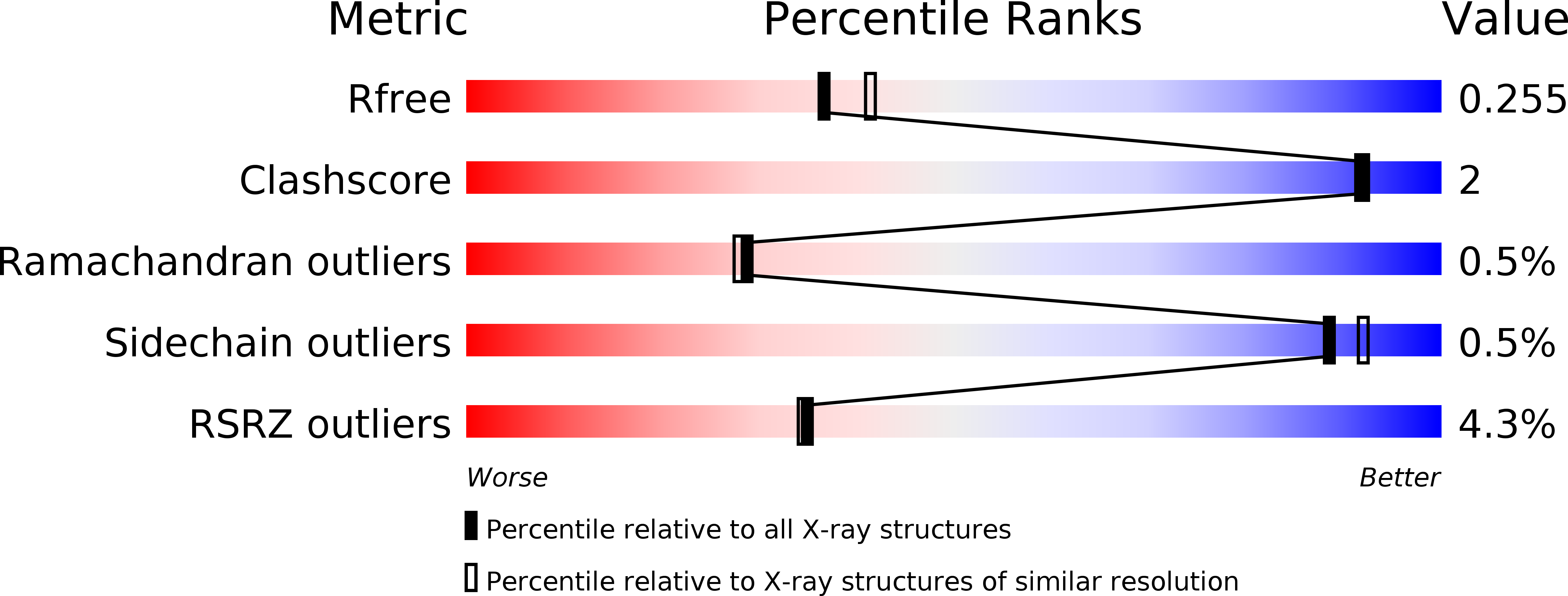
Resolution:
2.24 Å
R-Value Free:
0.25
R-Value Work:
0.21
R-Value Observed:
0.21
Space Group:
P 21 21 2