Deposition Date
2019-01-09
Release Date
2019-12-25
Last Version Date
2024-10-23
Entry Detail
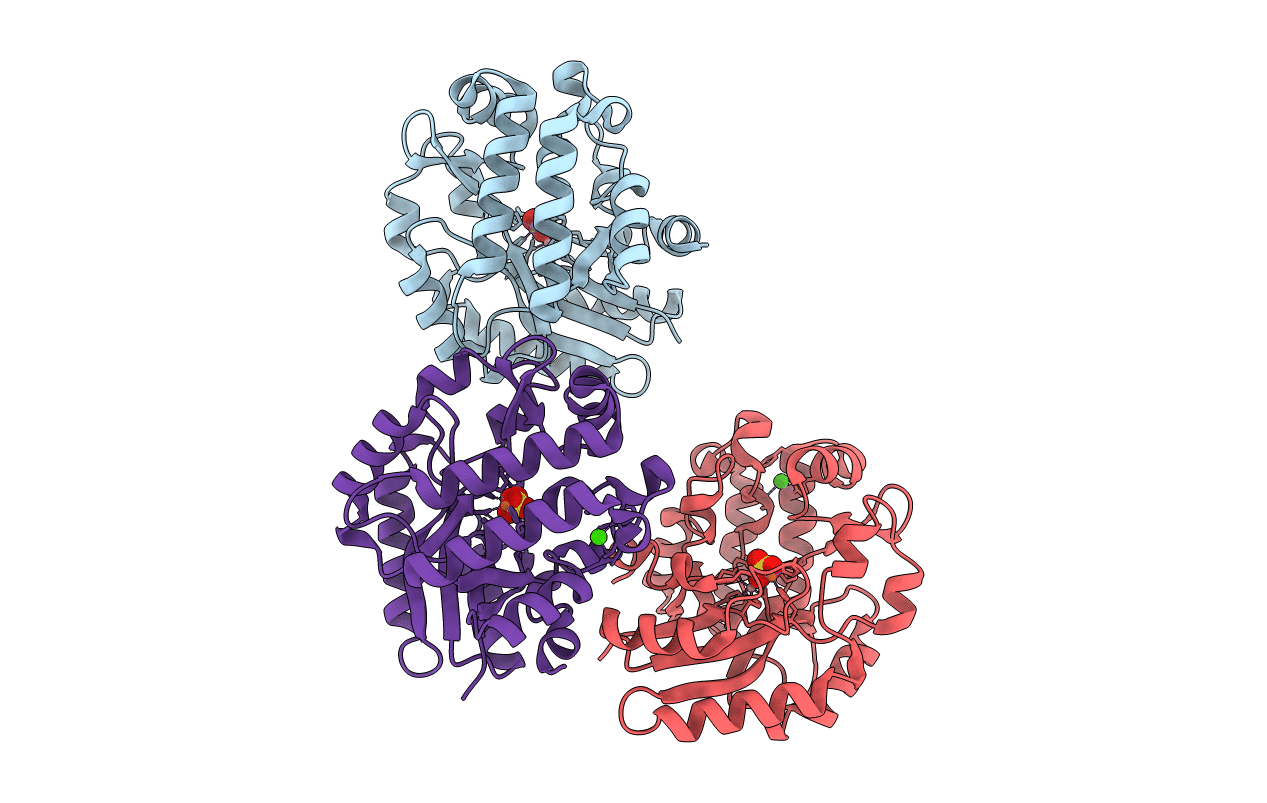
PDB ID:
6NLR
Keywords:
Title:
Crystal structure of the putative histidinol phosphatase hisK from Listeria monocytogenes with trinuclear metals determined by PIXE revealing sulphate ion in active site. Based on PIXE analysis and original date from 3DCP
Biological Source:
Source Organism(s):
Listeria monocytogenes serotype 4b str. H7858 (Taxon ID: 267410)
Expression System(s):
Method Details:
Experimental Method:
Resolution:
2.10 Å
R-Value Free:
0.18
R-Value Work:
0.15
R-Value Observed:
0.15
Space Group:
P 64


