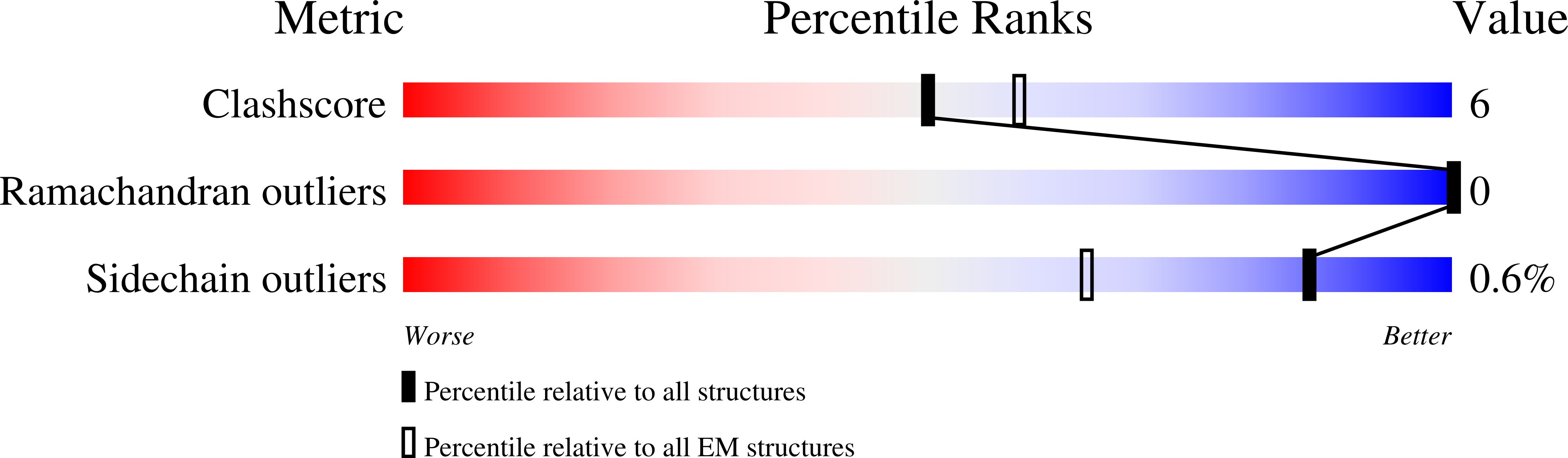
Deposition Date
2019-01-03
Release Date
2019-02-20
Last Version Date
2024-03-20
Entry Detail
PDB ID:
6NJP
Keywords:
Title:
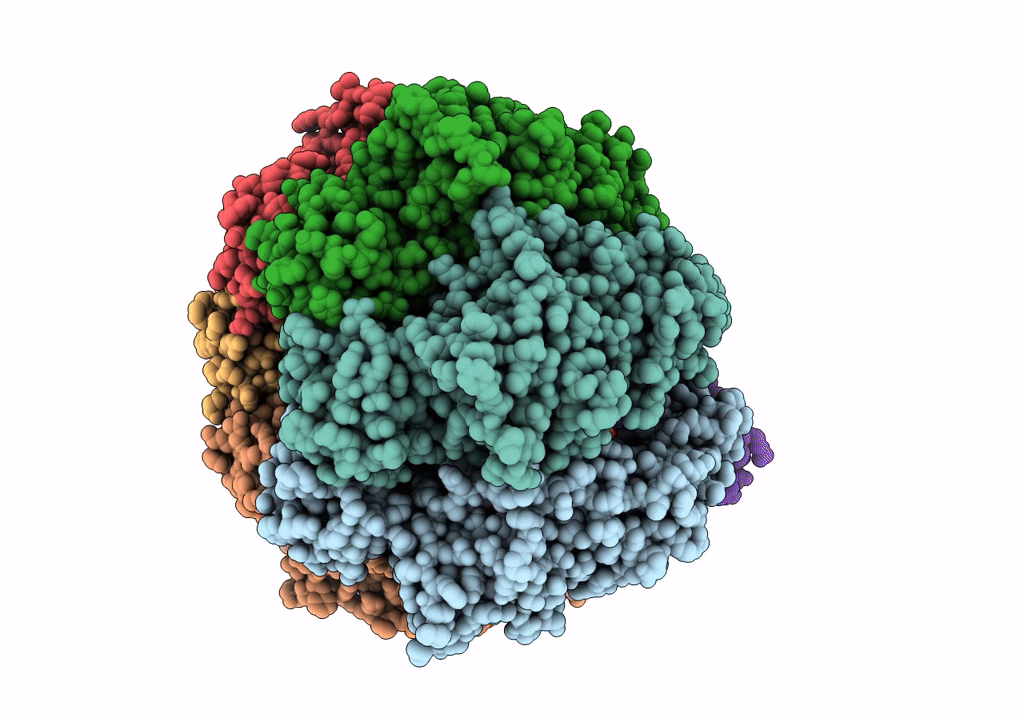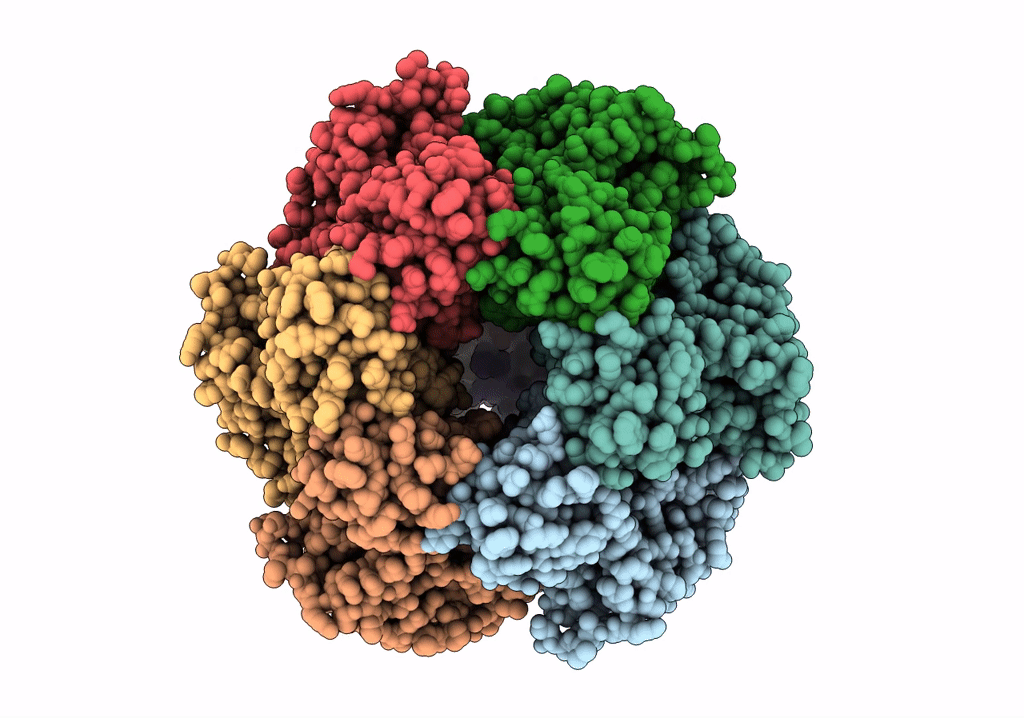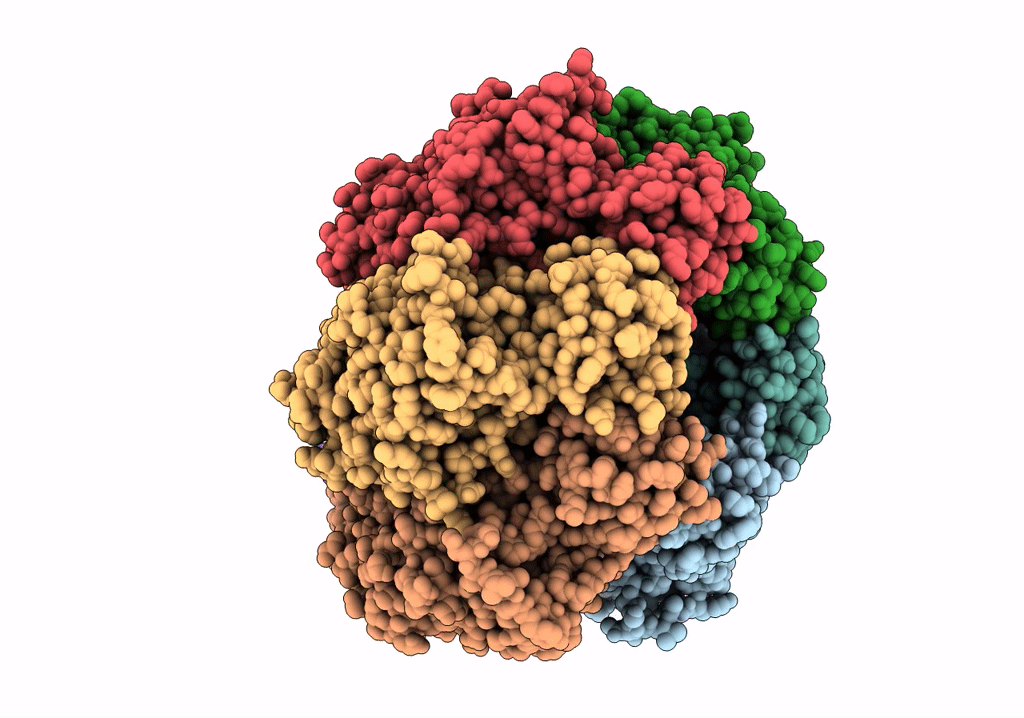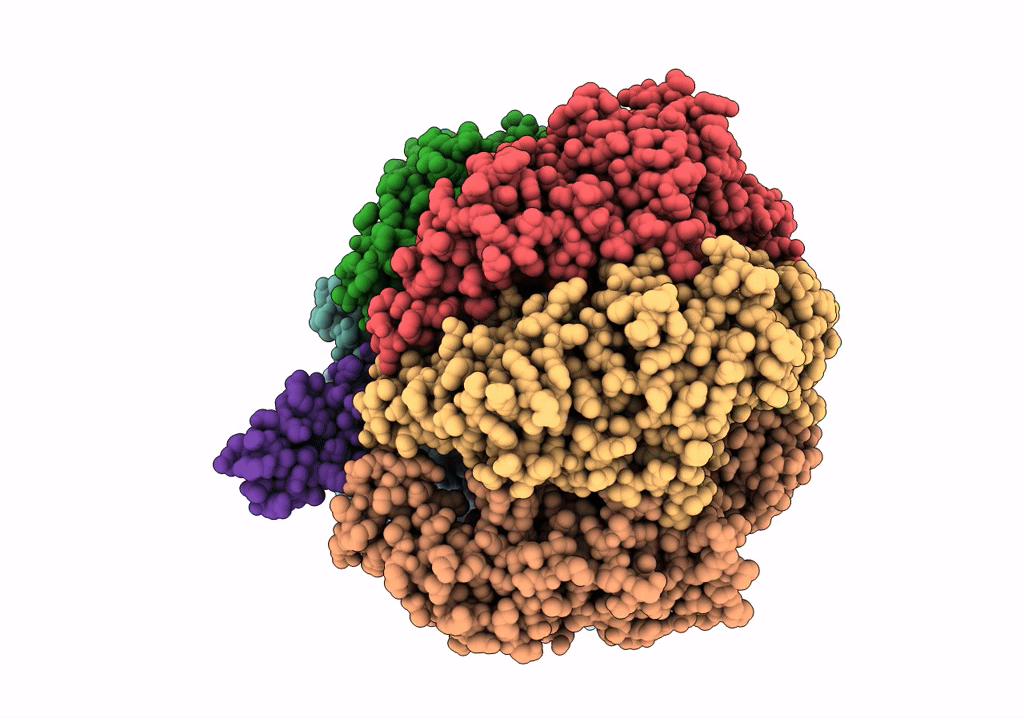
Structure of the assembled ATPase EscN in complex with its central stalk EscO from the enteropathogenic E. coli (EPEC) type III secretion system
Biological Source:
Source Organism(s):
Expression System(s):
Method Details:
Experimental Method:
Resolution:
3.29 Å
Aggregation State:
PARTICLE
Reconstruction Method:
SINGLE PARTICLE