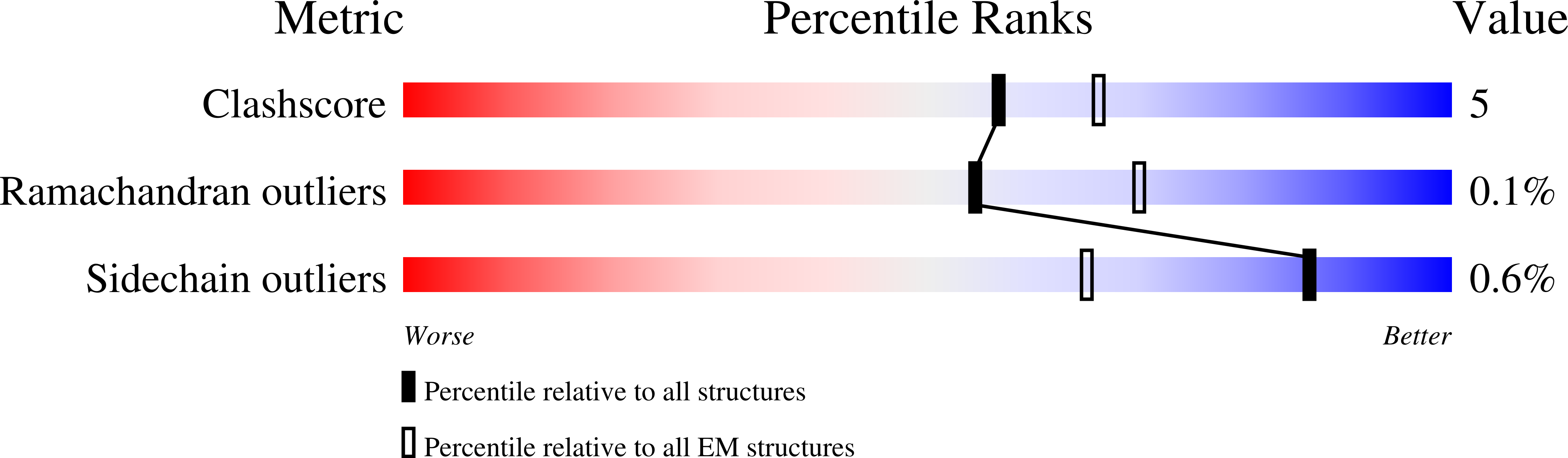Deposition Date
2019-01-03
Release Date
2019-04-24
Last Version Date
2024-12-25
Entry Detail
PDB ID:
6NJM
Keywords:
Title:
Architecture and subunit arrangement of native AMPA receptors
Biological Source:
Source Organism(s):
Mus musculus (Taxon ID: 10090)
Rattus norvegicus (Taxon ID: 10116)
Rattus norvegicus (Taxon ID: 10116)
Expression System(s):
Method Details:
Experimental Method:
Resolution:
6.50 Å
Aggregation State:
PARTICLE
Reconstruction Method:
SINGLE PARTICLE