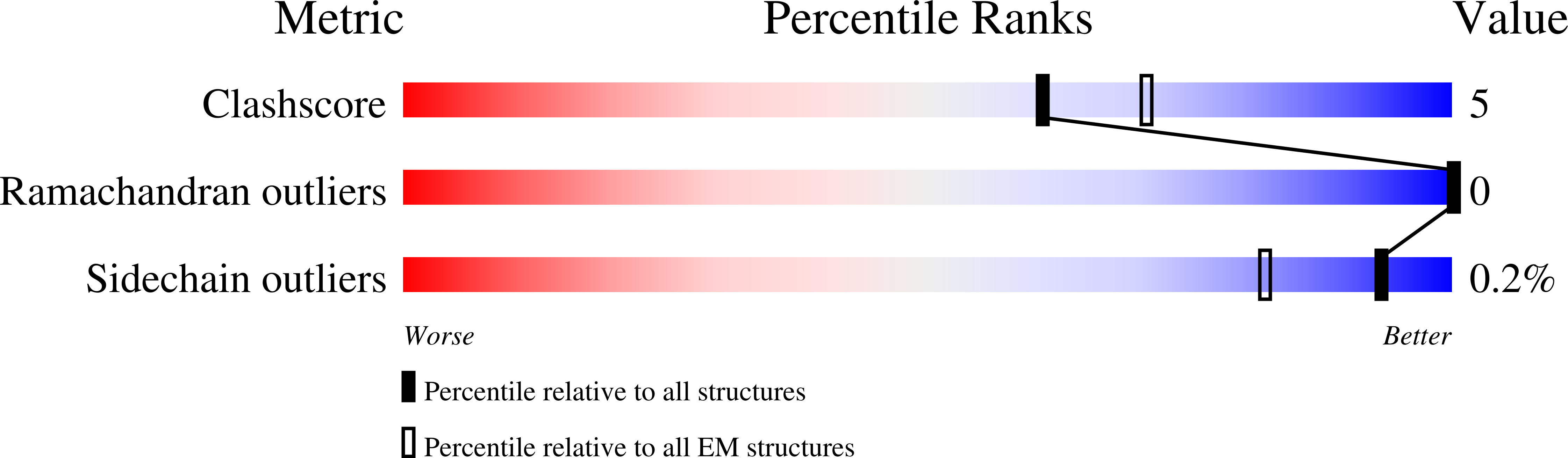
Deposition Date
2018-12-04
Release Date
2019-03-13
Last Version Date
2024-03-20
Entry Detail
PDB ID:
6N9Y
Keywords:
Title:
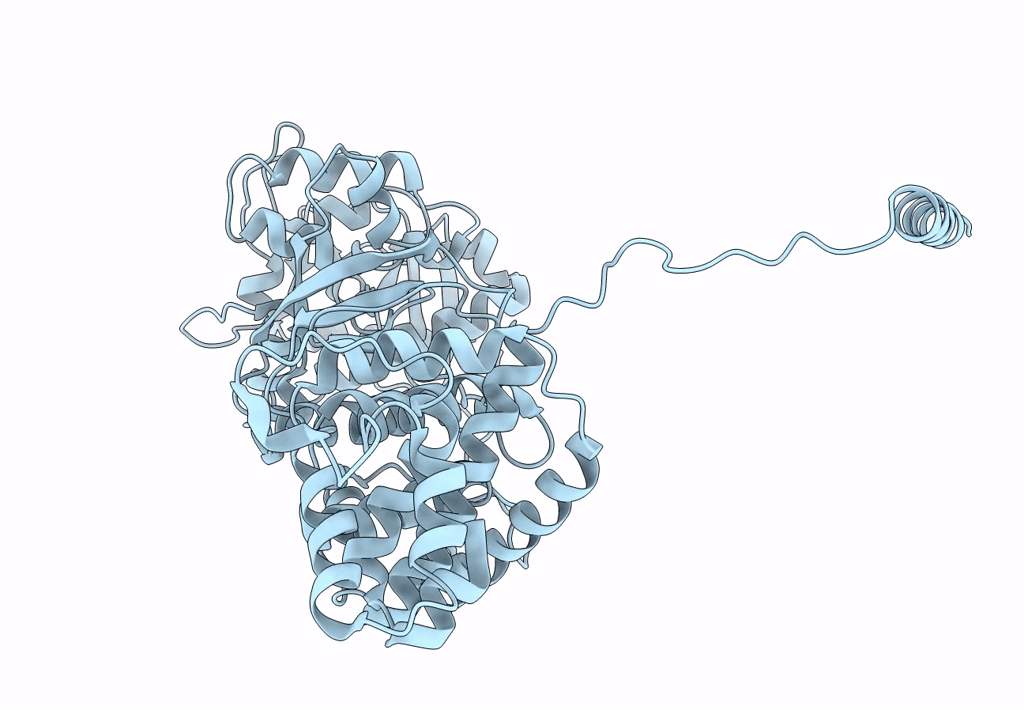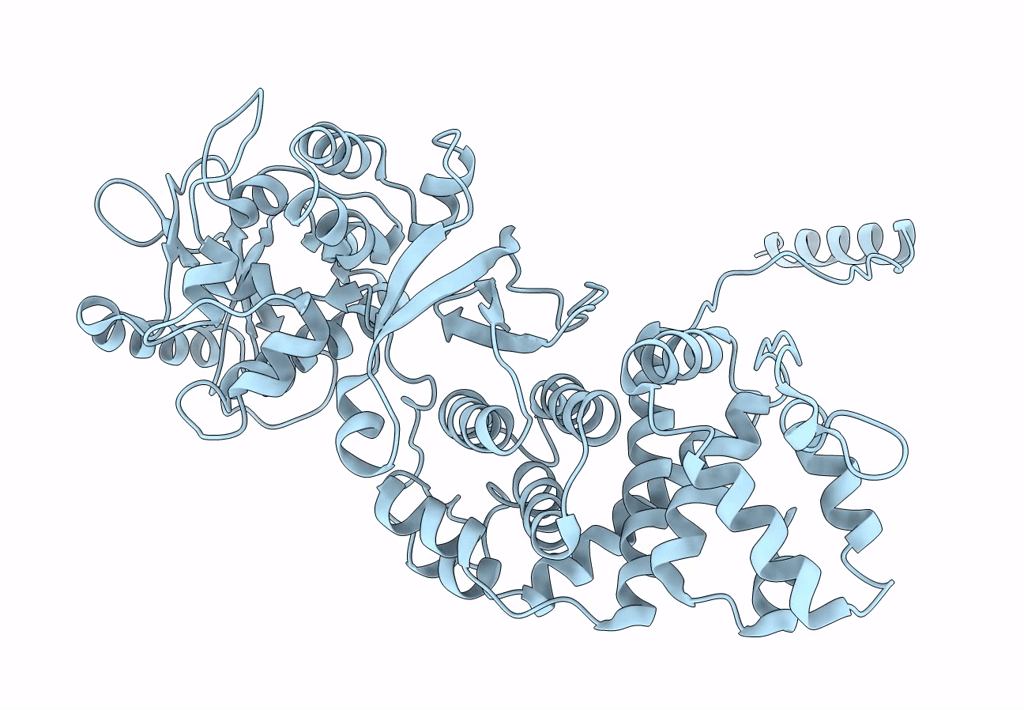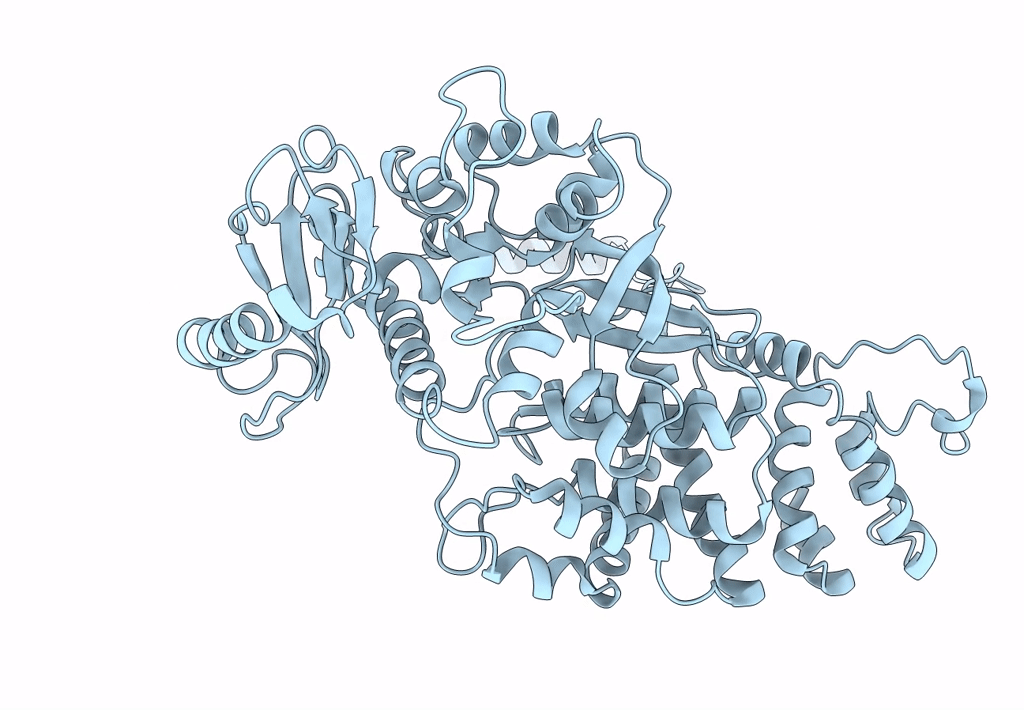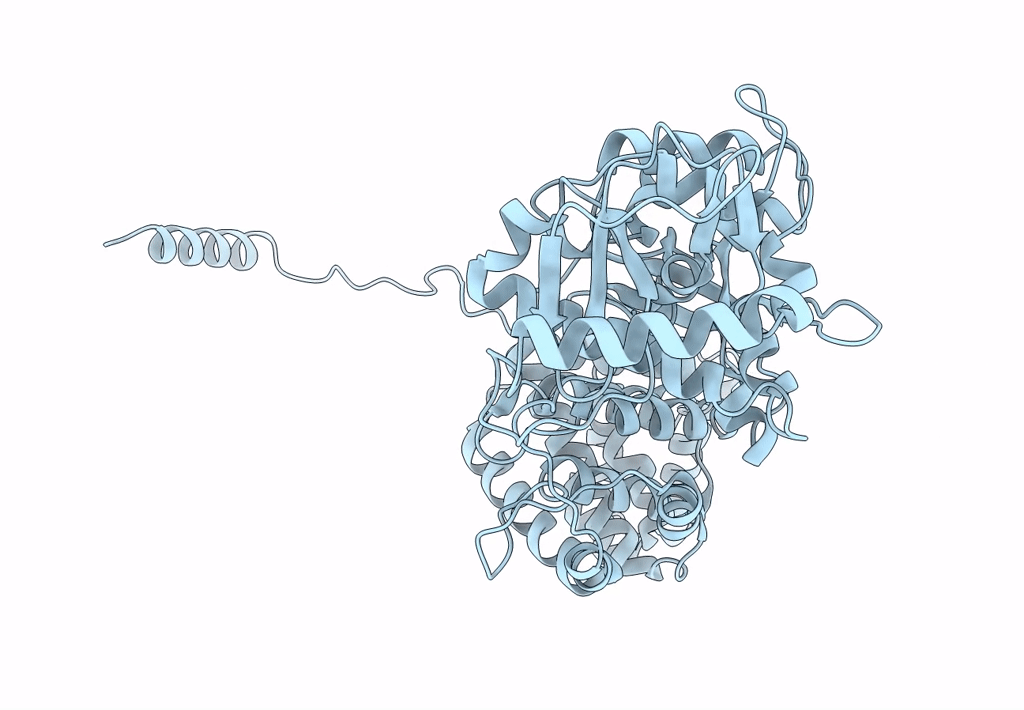
Atomic structure of Non-Structural protein 1 of bluetongue virus
Biological Source:
Source Organism:
Bluetongue virus 23 (Taxon ID: 45031)
Method Details:
Experimental Method:
Resolution:
4.00 Å
Aggregation State:
HELICAL ARRAY
Reconstruction Method:
HELICAL