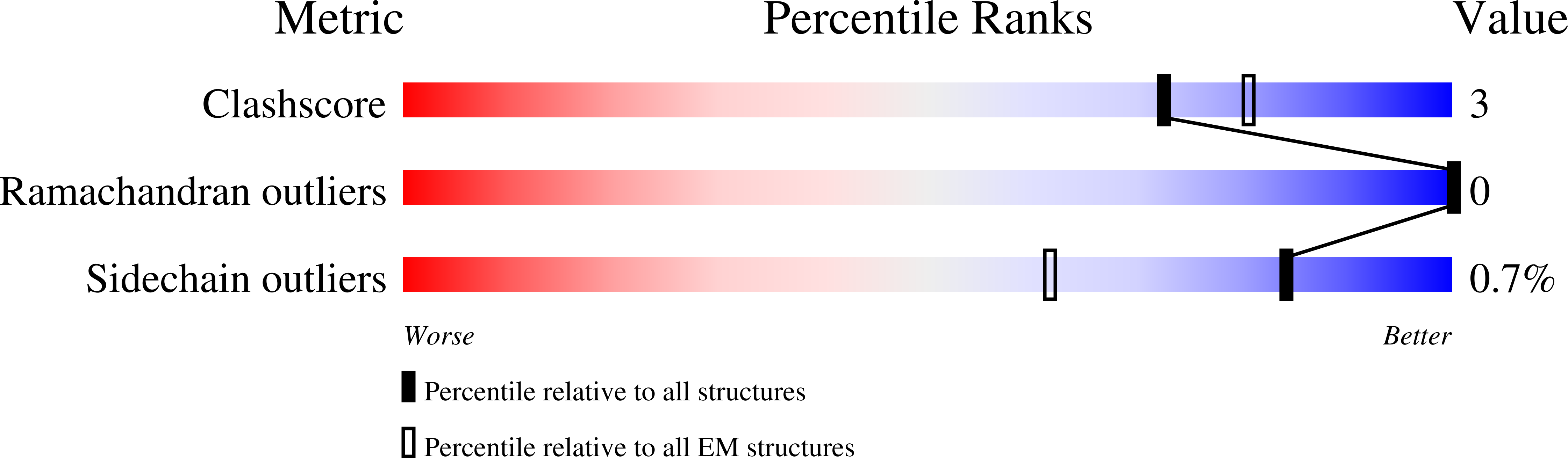
Deposition Date
2018-11-28
Release Date
2019-03-06
Last Version Date
2024-03-20
Entry Detail
PDB ID:
6N7V
Keywords:
Title:
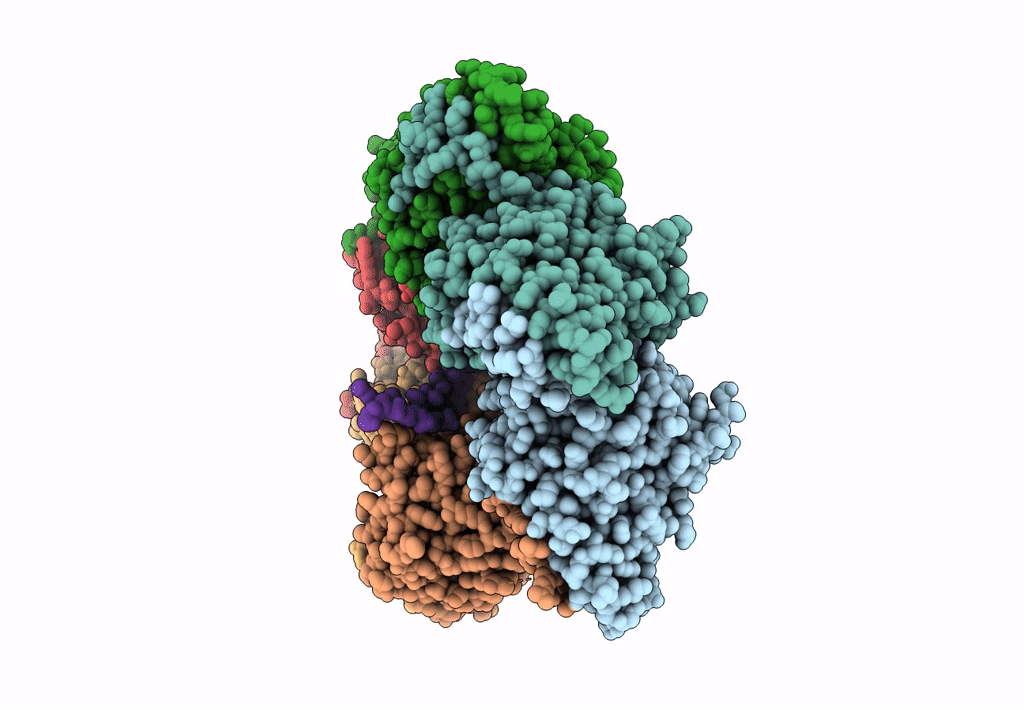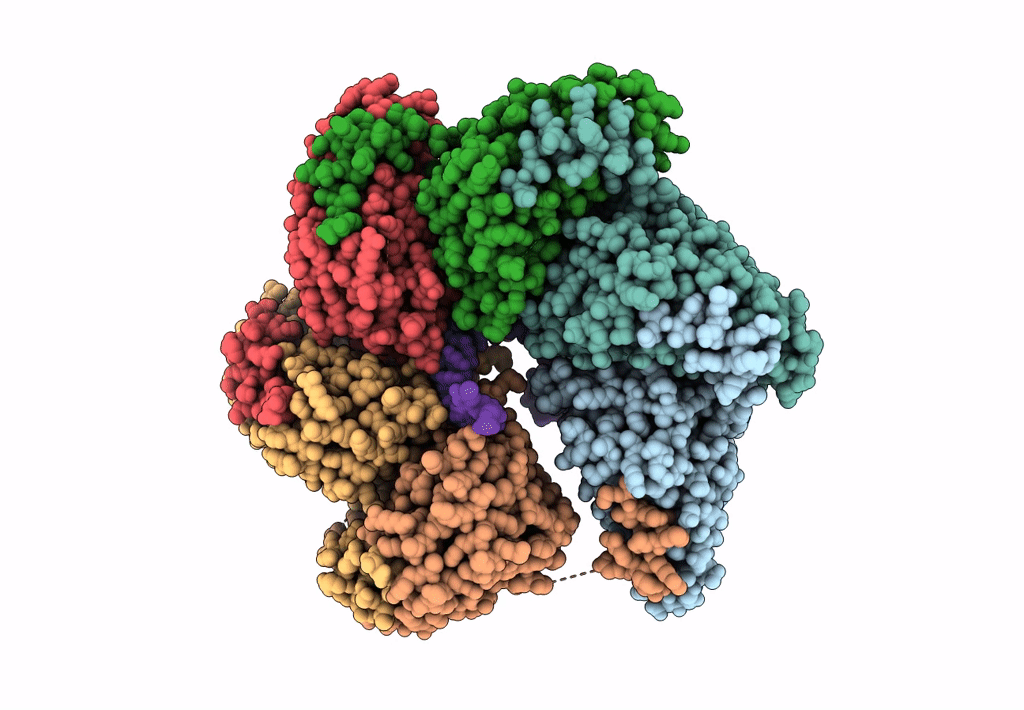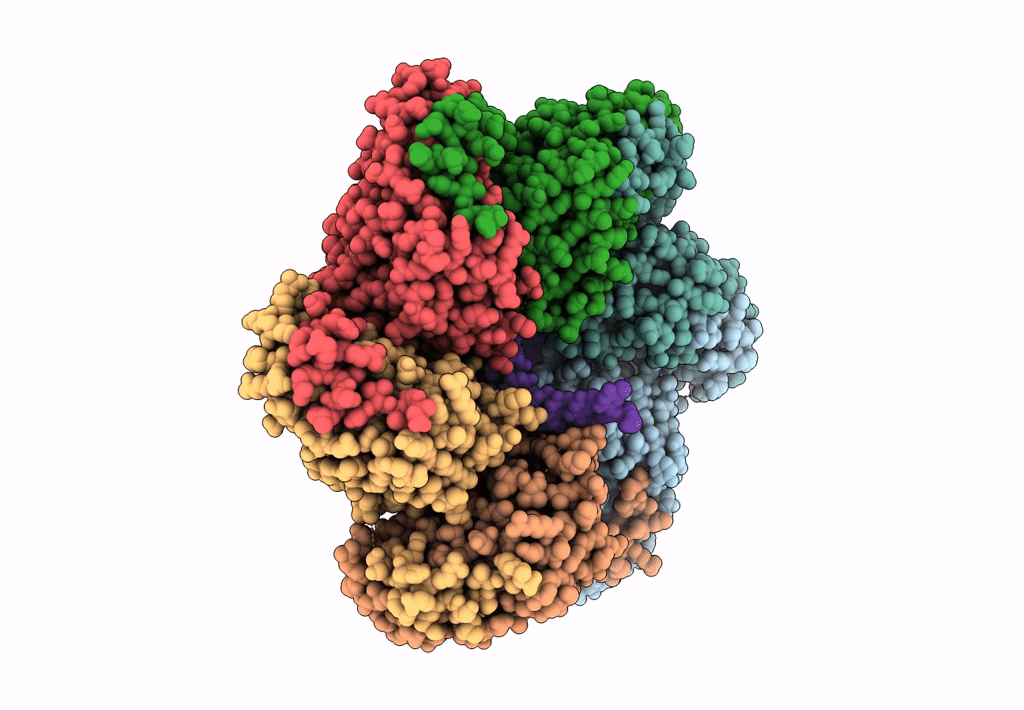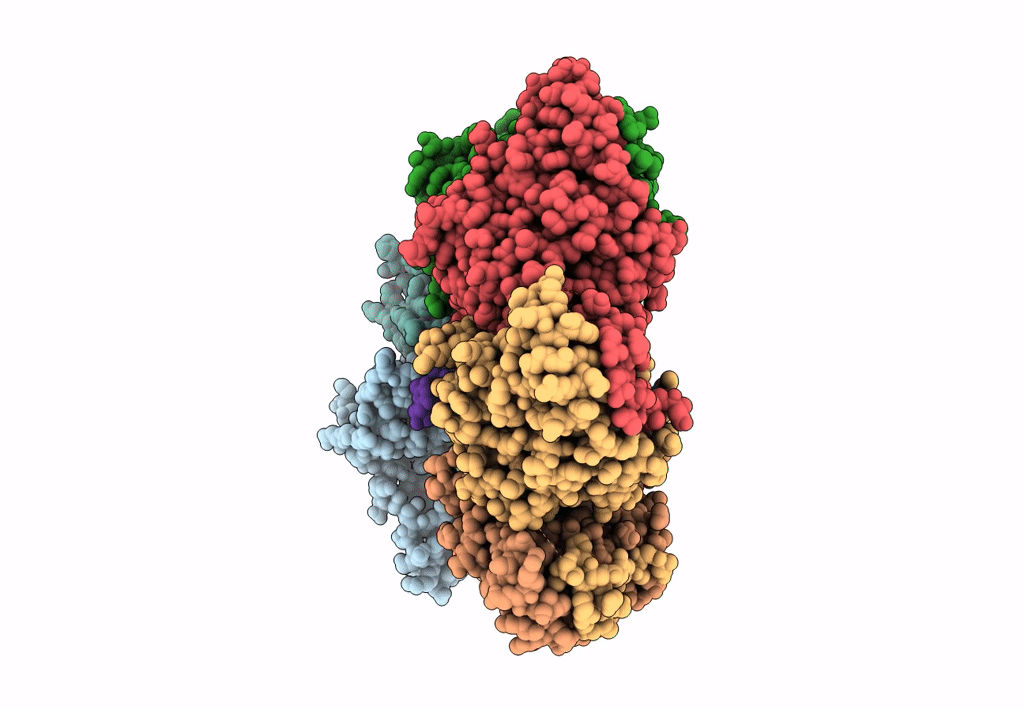
Structure of bacteriophage T7 gp4 (helicase-primase, E343Q mutant) in complex with ssDNA, dTTP, AC dinucleotide, and CTP (from multiple lead complexes)
Biological Source:
Source Organism(s):
Enterobacteria phage T7 (Taxon ID: 10760)
Expression System(s):
Method Details:
Experimental Method:
Resolution:
3.80 Å
Aggregation State:
PARTICLE
Reconstruction Method:
SINGLE PARTICLE