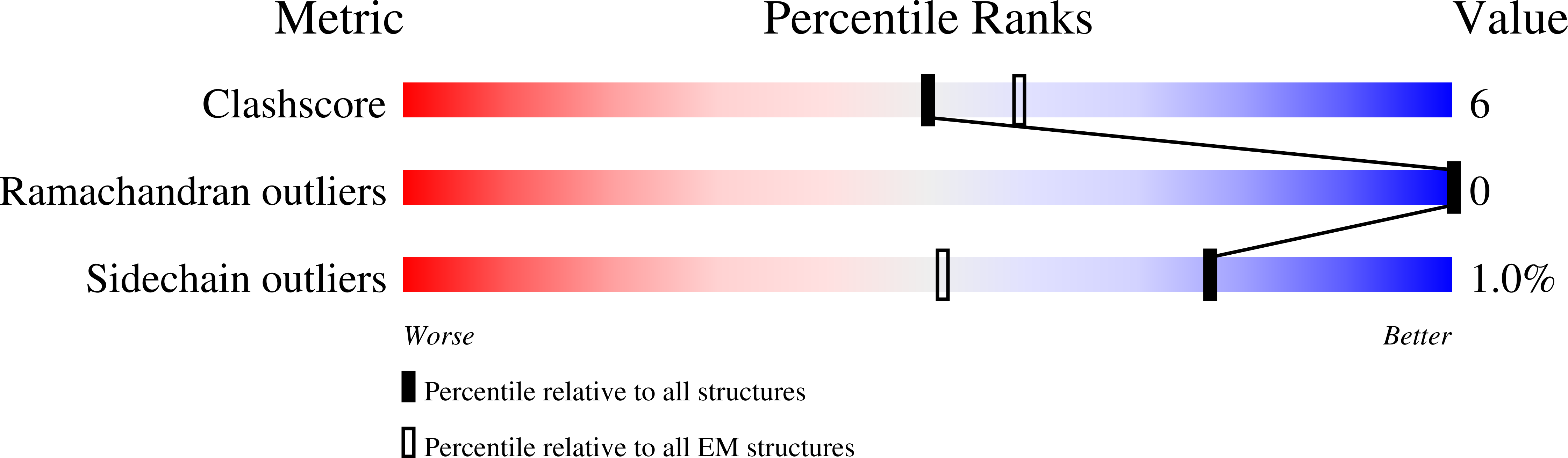
Deposition Date
2018-11-07
Release Date
2019-03-13
Last Version Date
2024-03-20
Entry Detail
PDB ID:
6N0G
Keywords:
Title:
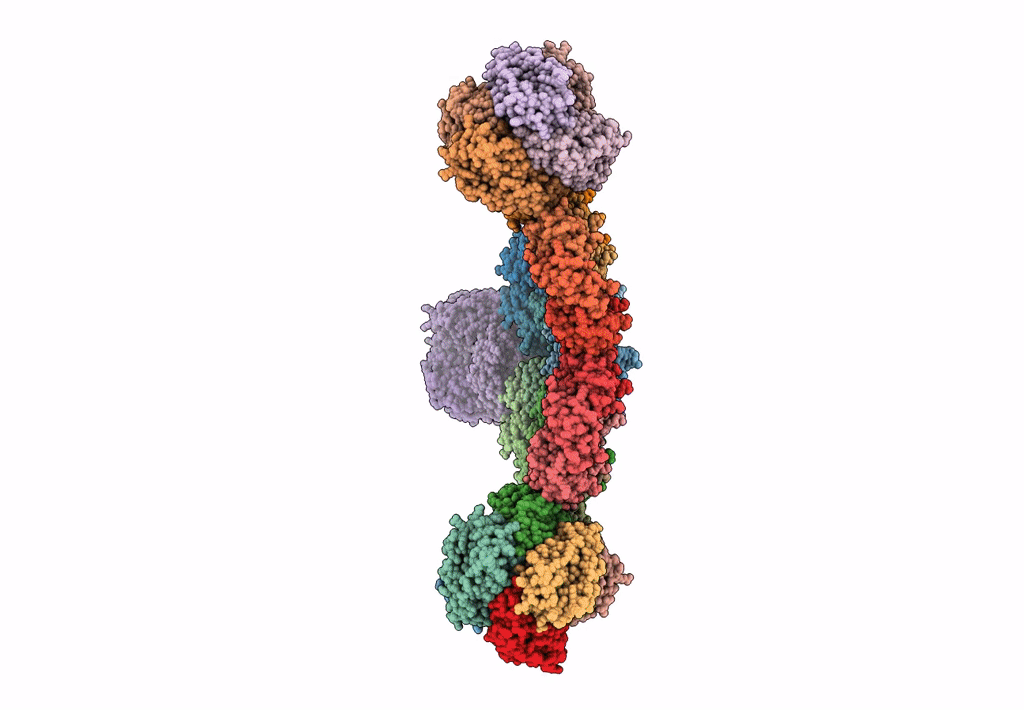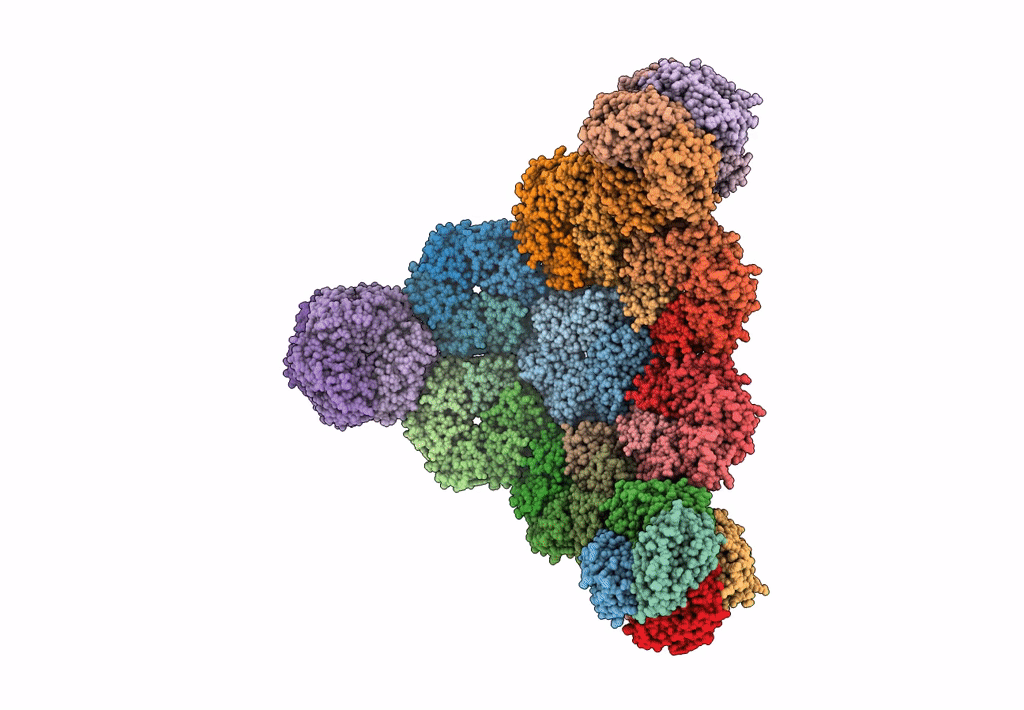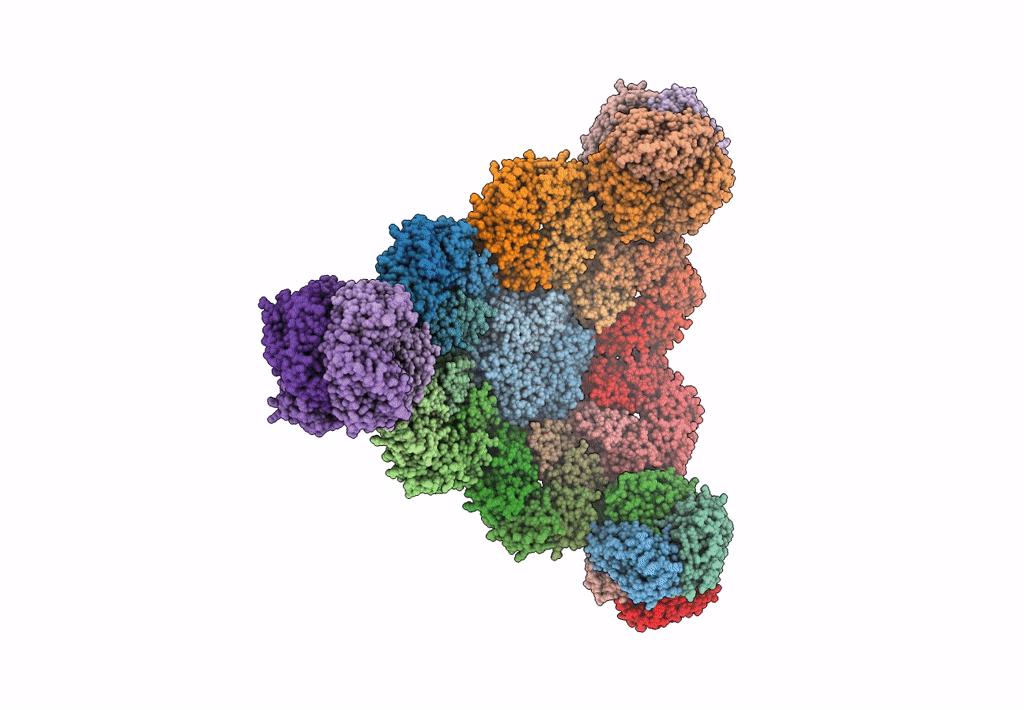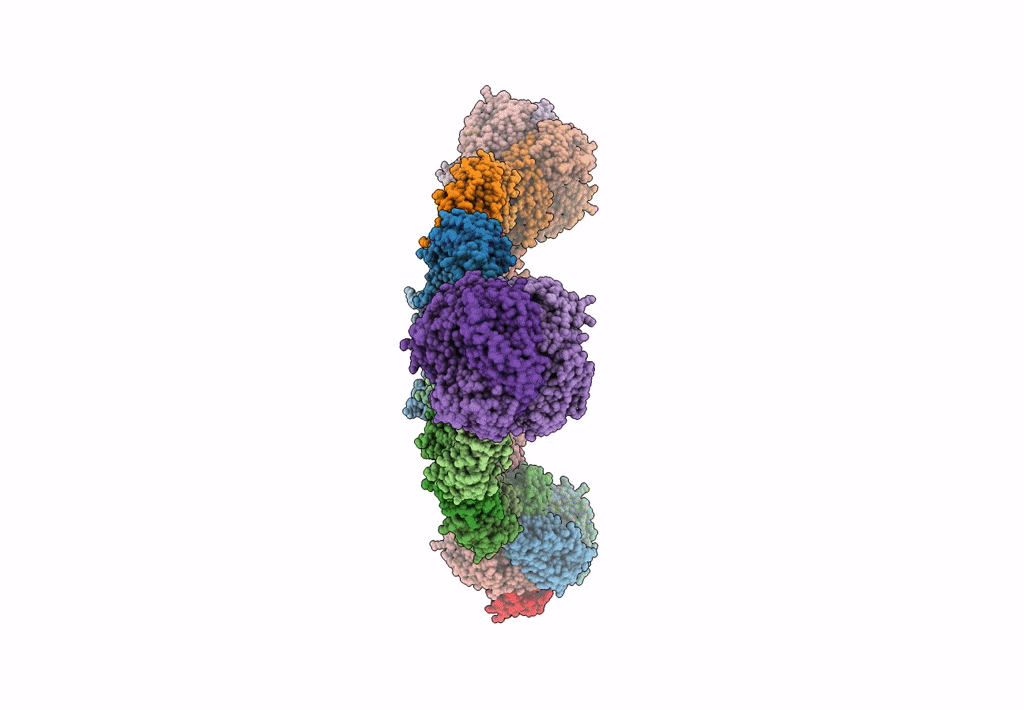
Cryo-EM structure of the HO BMC shell: subregion classified for BMC-T: TS-TDTDTD
Biological Source:
Source Organism:
Host Organism:
Method Details:
Experimental Method:
Resolution:
3.60 Å
Aggregation State:
PARTICLE
Reconstruction Method:
SINGLE PARTICLE