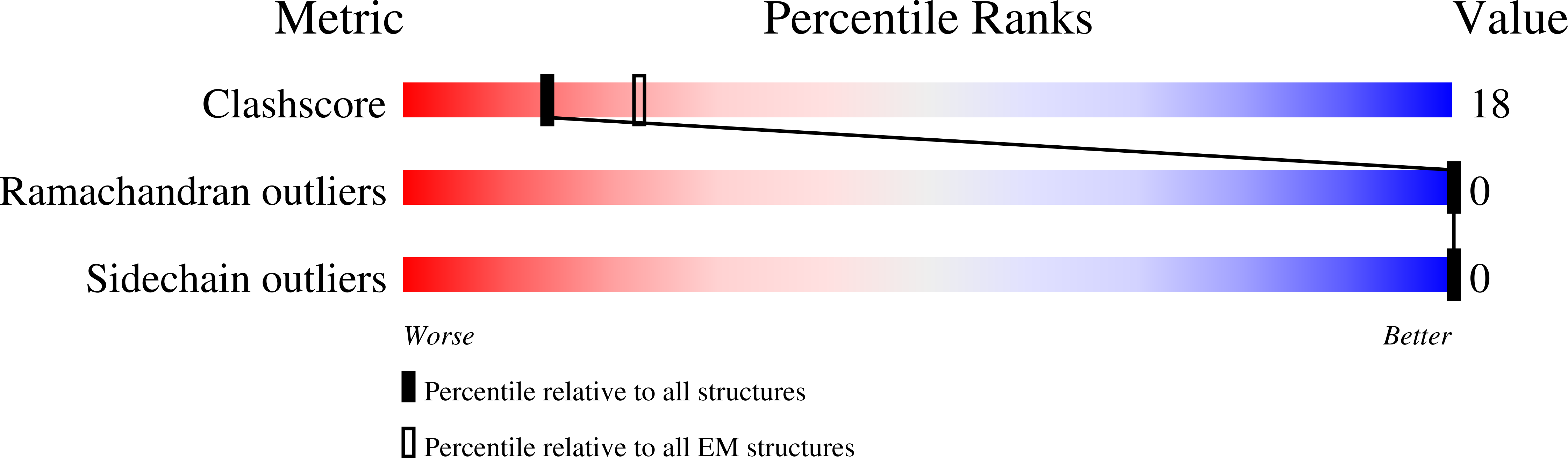
Deposition Date
2018-10-19
Release Date
2019-04-24
Last Version Date
2024-03-13
Entry Detail
PDB ID:
6MTI
Keywords:
Title:
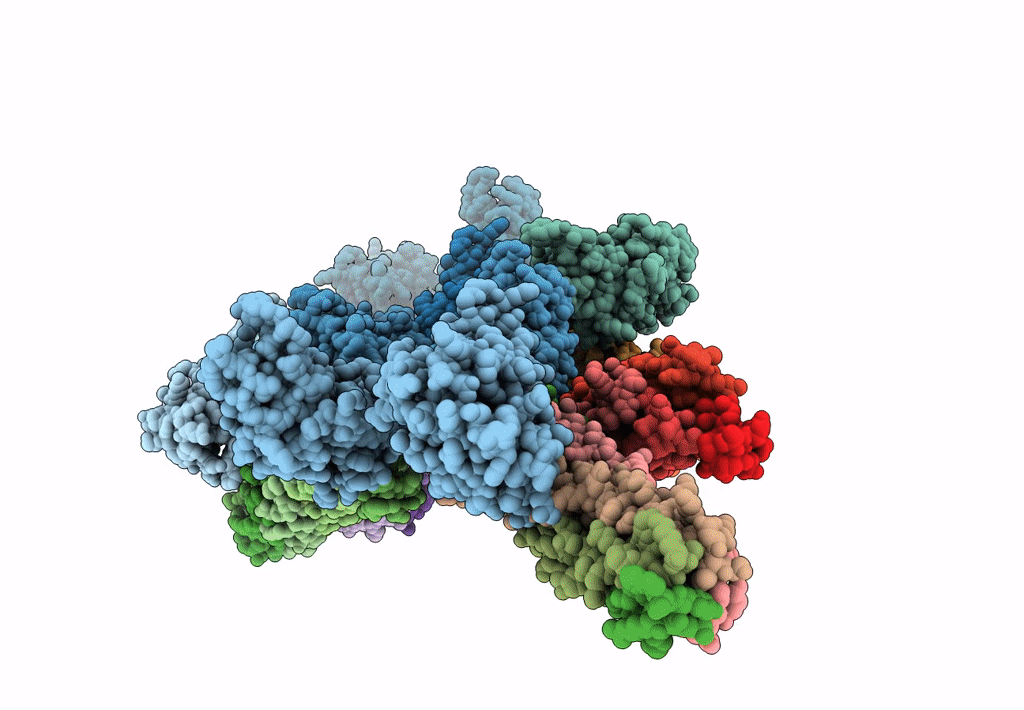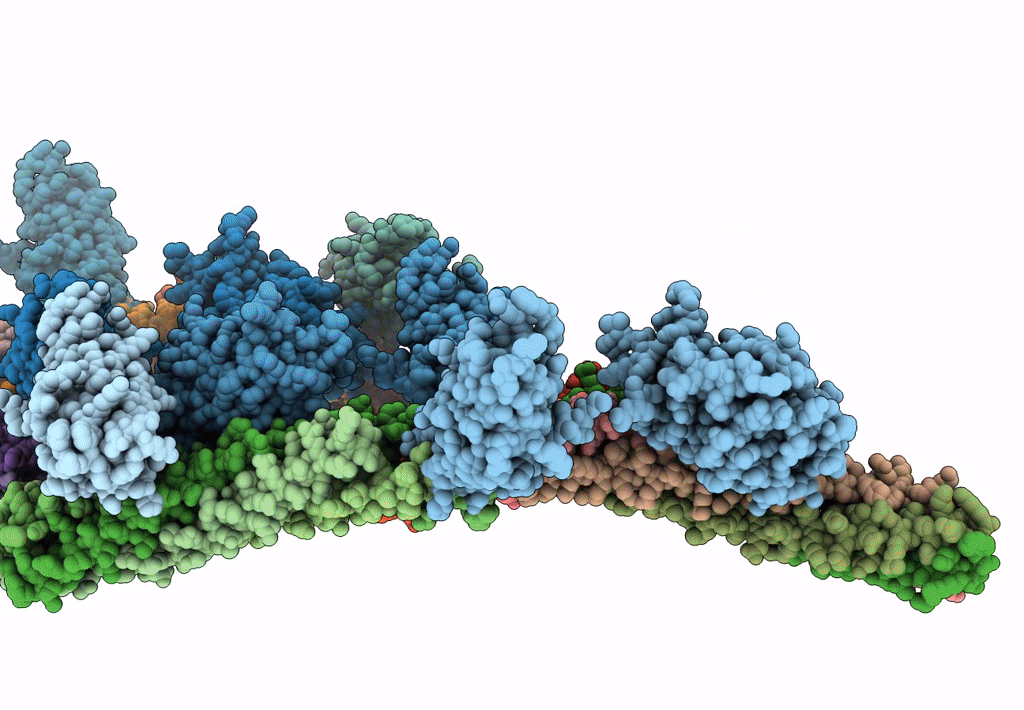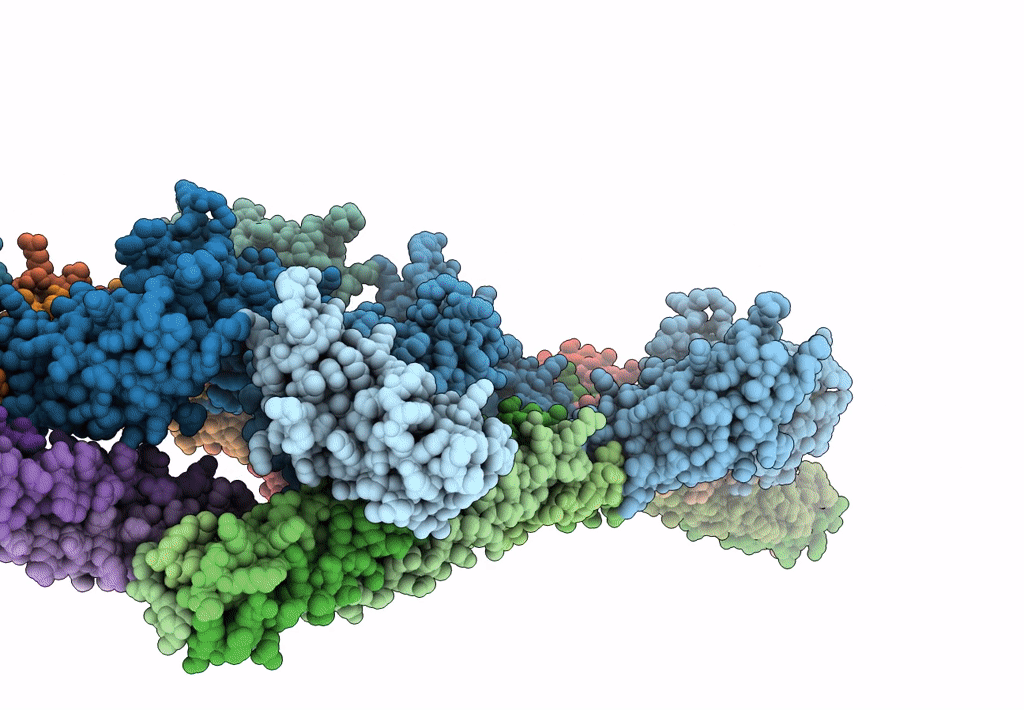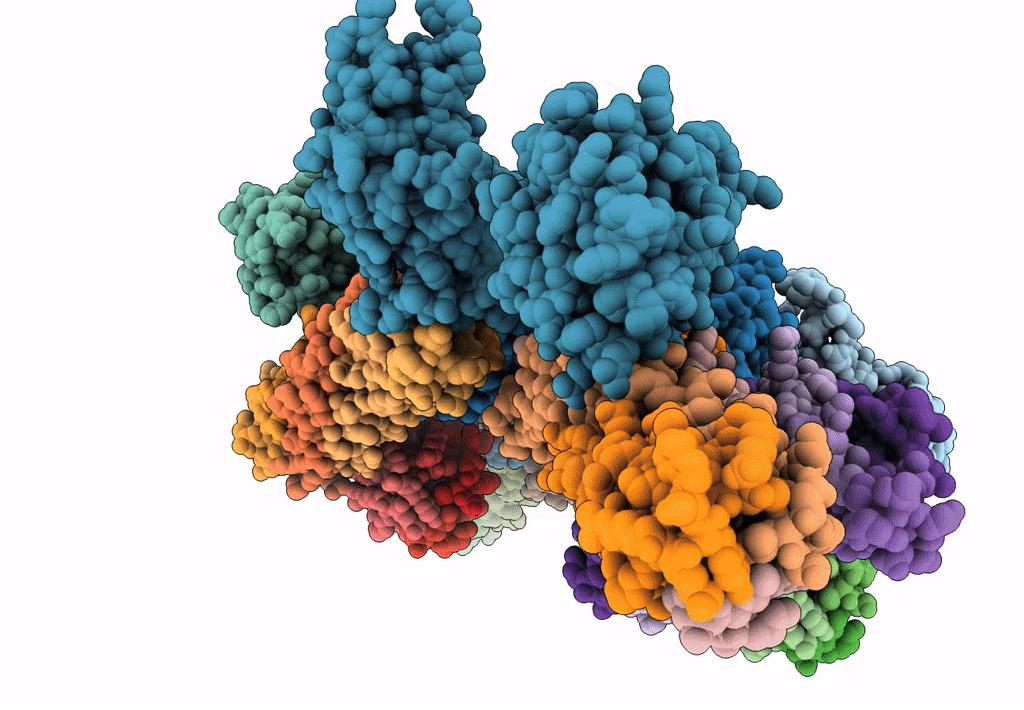
Synaptotagmin-1 C2A, C2B domains and SNARE-pin proteins (5CCI) individually docked into Cryo-EM map of C2AB-SNARE complexes helically organized on lipid nanotube surface in presence of Mg2+
Biological Source:
Source Organism(s):
Rattus norvegicus (Taxon ID: 10116)
Expression System(s):
Method Details:
Experimental Method:
Resolution:
10.40 Å
Aggregation State:
HELICAL ARRAY
Reconstruction Method:
HELICAL