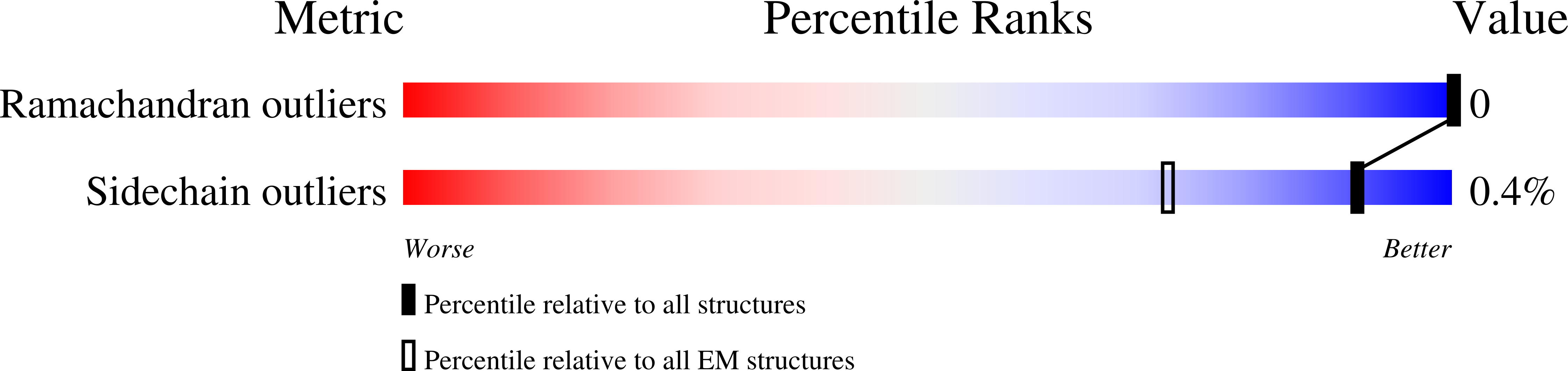Deposition Date
2018-10-06
Release Date
2019-07-24
Last Version Date
2024-11-20
Entry Detail
PDB ID:
6MPG
Keywords:
Title:
Cryo-EM structure at 3.2 A resolution of HIV-1 fusion peptide-directed antibody, A12V163-b.01, elicited by vaccination of Rhesus macaques, in complex with stabilized HIV-1 Env BG505 DS-SOSIP, which was also bound to antibodies VRC03 and PGT122
Biological Source:
Source Organism(s):
Macaca mulatta (Taxon ID: 9544)
Human immunodeficiency virus 1 (Taxon ID: 11676)
Homo sapiens (Taxon ID: 9606)
Human immunodeficiency virus 1 (Taxon ID: 11676)
Homo sapiens (Taxon ID: 9606)
Expression System(s):
Method Details:
Experimental Method:
Resolution:
3.20 Å
Aggregation State:
PARTICLE
Reconstruction Method:
SINGLE PARTICLE