Deposition Date
2019-12-20
Release Date
2020-10-21
Last Version Date
2024-10-23
Entry Detail
PDB ID:
6LKS
Keywords:
Title:
Effects of zinc ion on oligomerization and pH stability of influenza virus hemagglutinin
Biological Source:
Source Organism(s):
Influenza A virus (A/Thailand/CU44/2006(H1N1)) (Taxon ID: 457457)
Expression System(s):
Method Details:
Experimental Method:
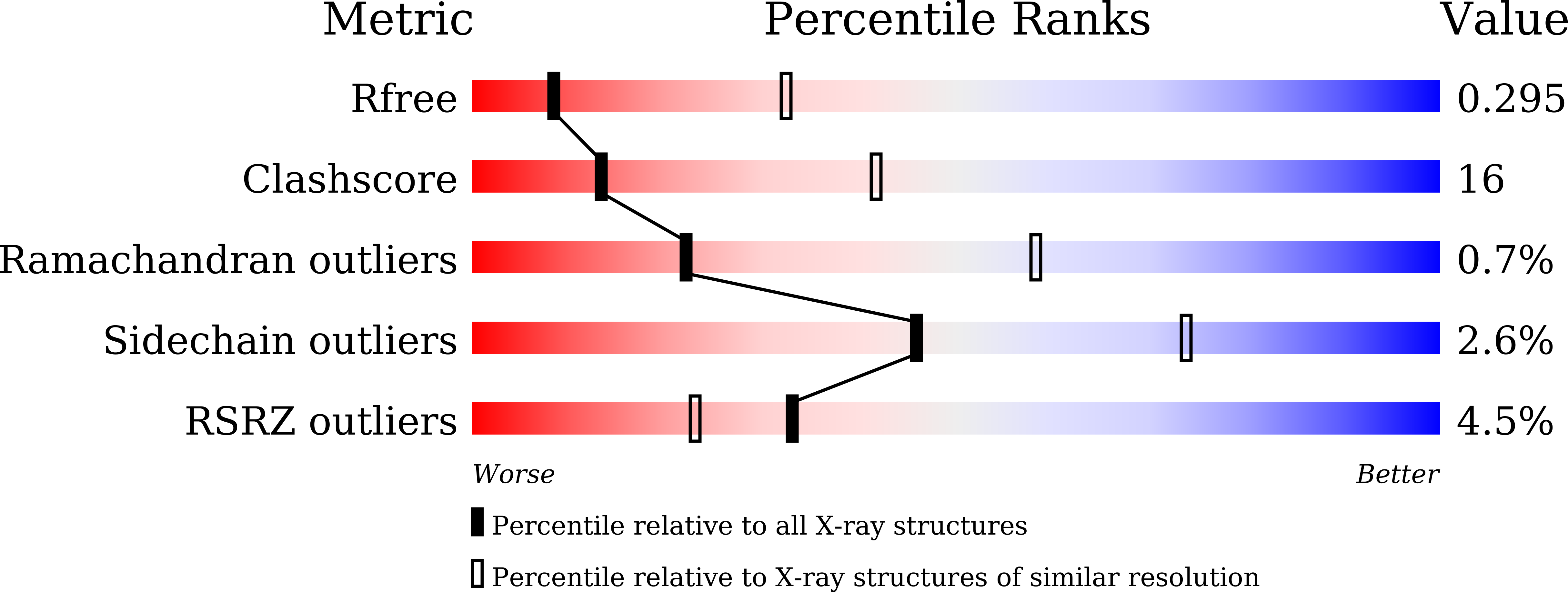
Resolution:
3.24 Å
R-Value Free:
0.29
R-Value Work:
0.26
R-Value Observed:
0.26
Space Group:
C 1 2 1