Deposition Date
2019-04-26
Release Date
2020-03-04
Last Version Date
2024-11-13
Entry Detail
PDB ID:
6JY8
Keywords:
Title:
Structure of dark-state marine bacterial chloride importer, NM-R3, with CW laser (ND-3%) at 95K.
Biological Source:
Source Organism:
Nonlabens marinus S1-08 (Taxon ID: 1454201)
Host Organism:
Method Details:
Experimental Method:
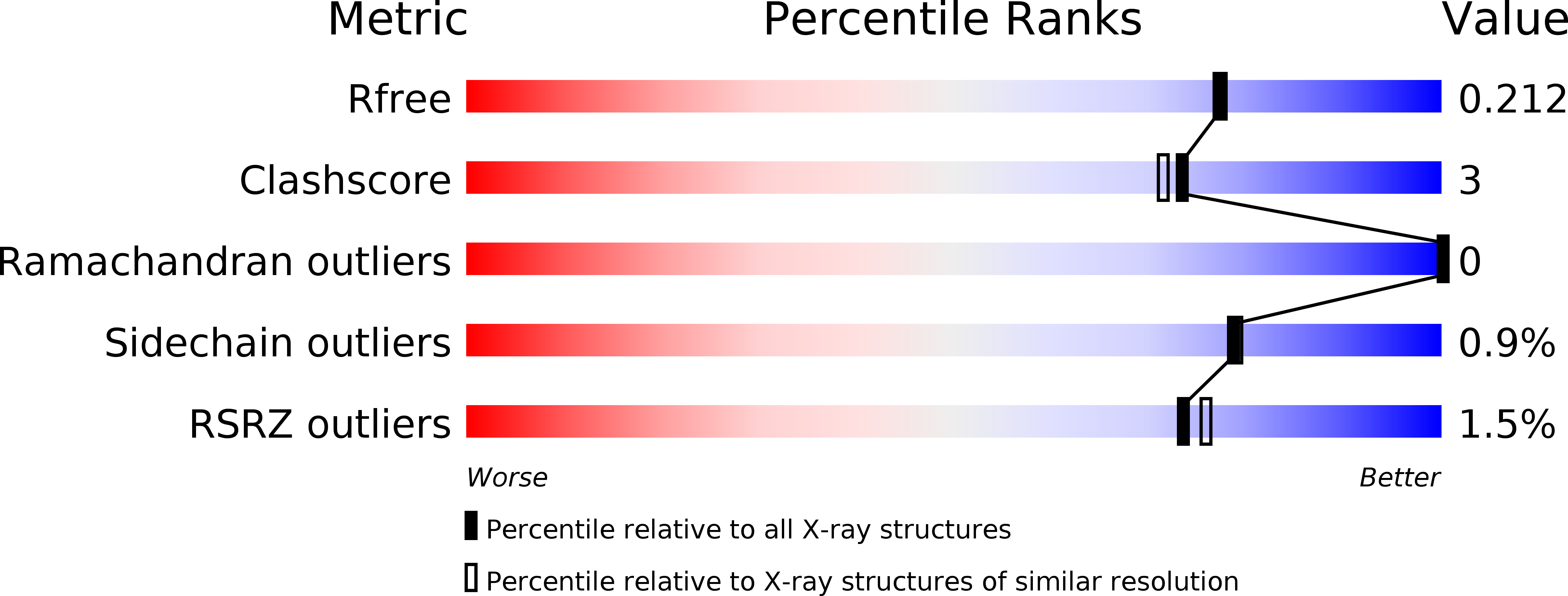
Resolution:
1.90 Å
R-Value Free:
0.21
R-Value Work:
0.17
R-Value Observed:
0.17
Space Group:
C 1 2 1