Deposition Date
2019-04-08
Release Date
2020-02-19
Last Version Date
2024-11-20
Entry Detail
PDB ID:
6JSJ
Keywords:
Title:
Structural analysis of a trimeric assembly of the mitochondrial dynamin-like GTPase Mgm1
Biological Source:
Source Organism(s):
Saccharomyces cerevisiae S288c (Taxon ID: 559292)
Expression System(s):
Method Details:
Experimental Method:
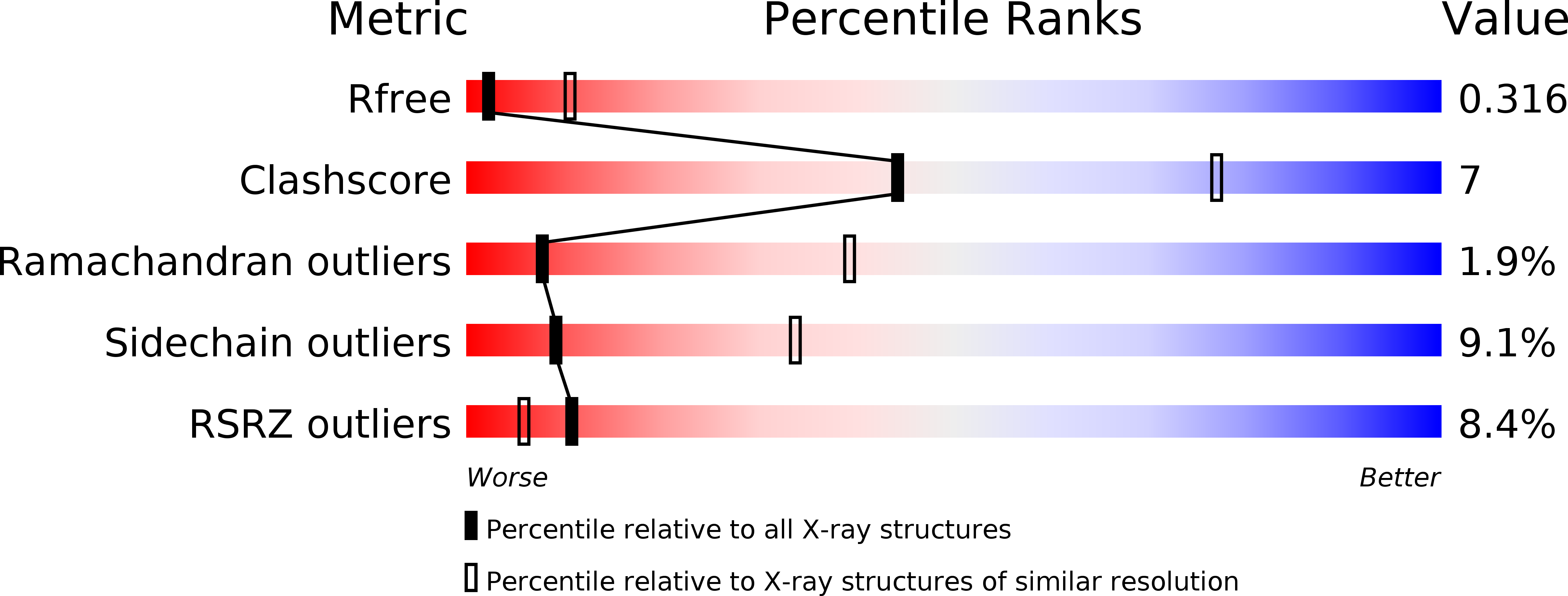
Resolution:
3.20 Å
R-Value Free:
0.30
R-Value Work:
0.24
R-Value Observed:
0.24
Space Group:
P 41 21 2