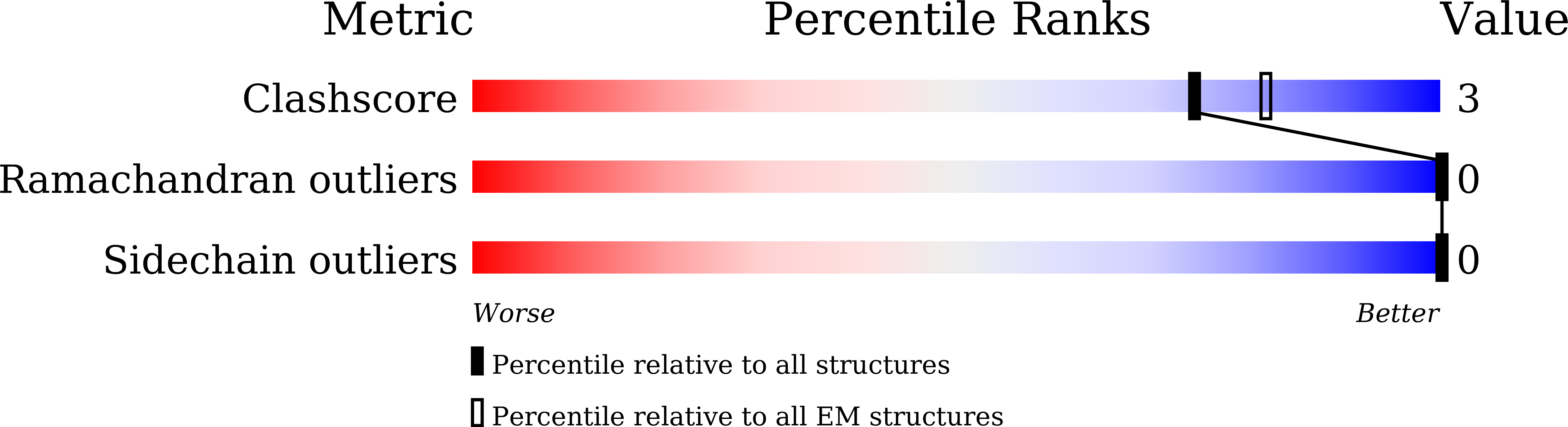
Deposition Date
2019-03-31
Release Date
2019-09-11
Last Version Date
2024-03-27
Entry Detail
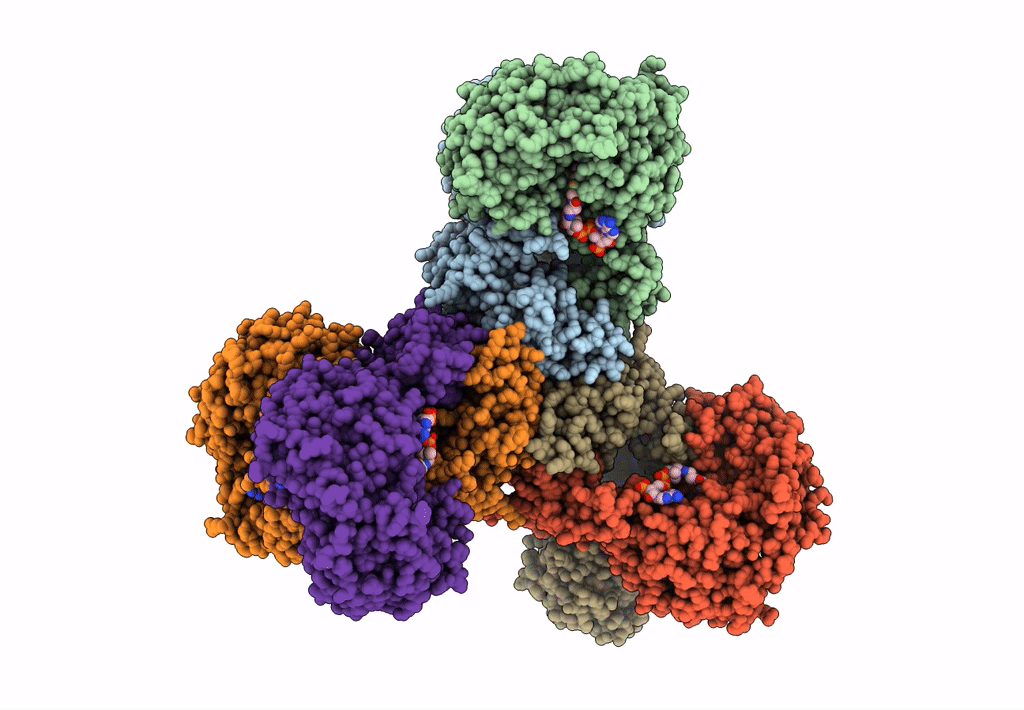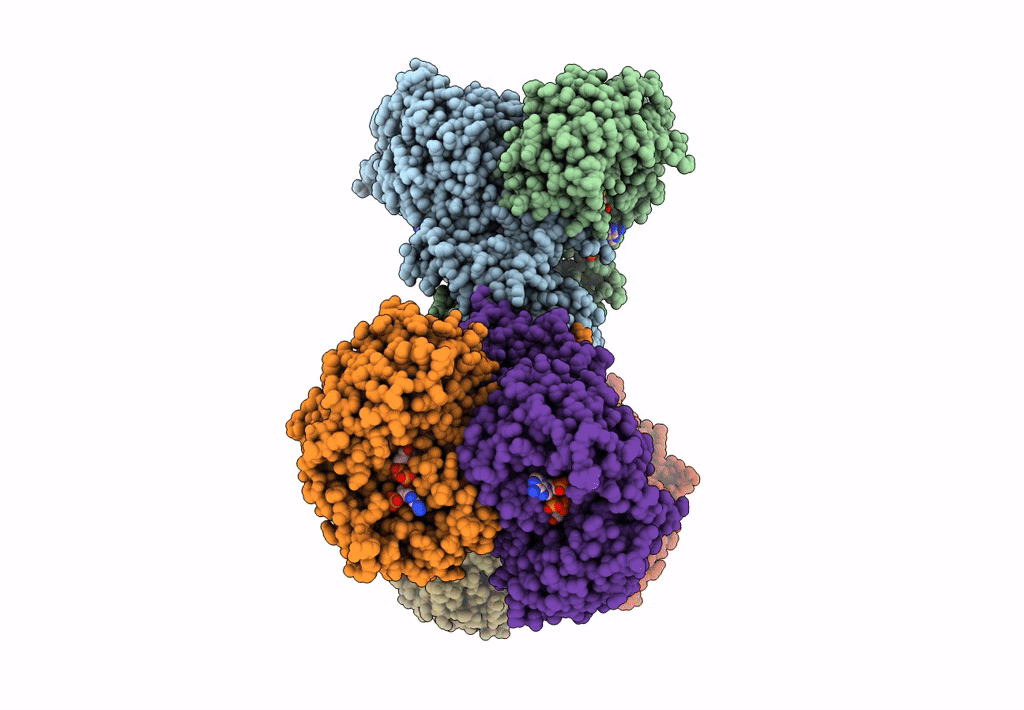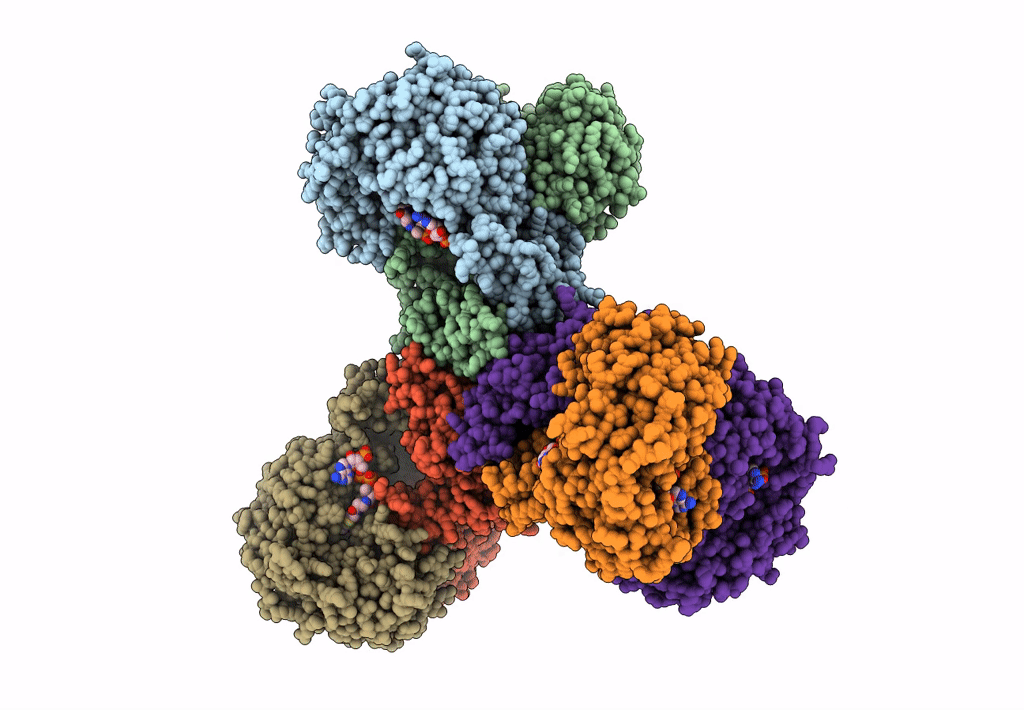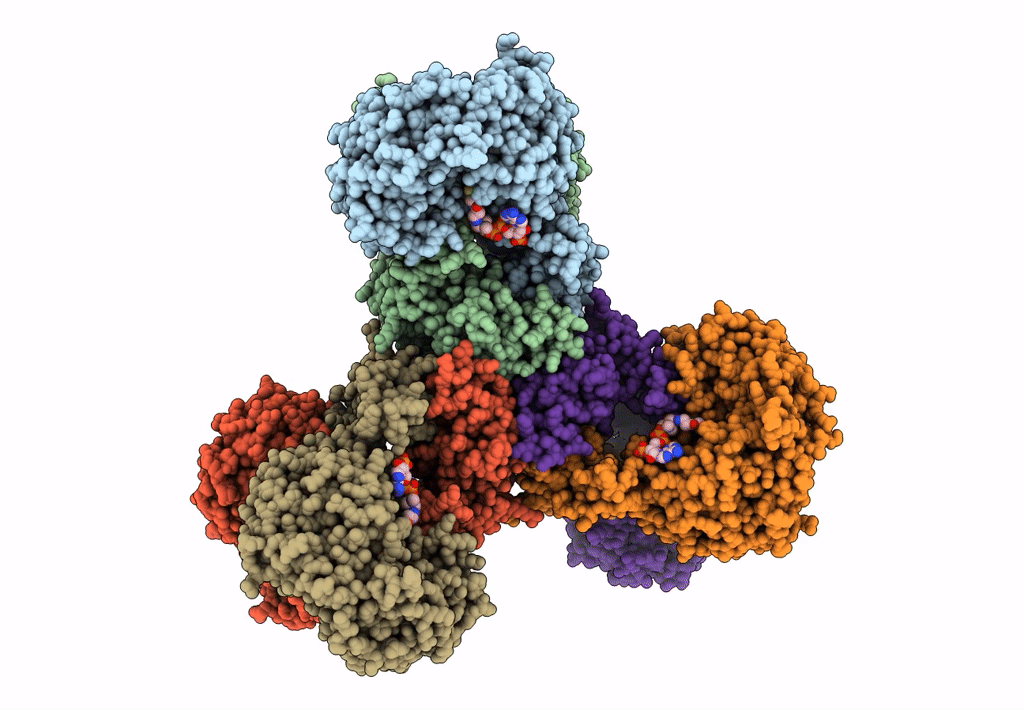
PDB ID:
6JQO
Keywords:
Title:
Structure of PaaZ, a bifunctional enzyme in complex with NADP+ and CCoA
Biological Source:
Source Organism:
Escherichia coli K-12 (Taxon ID: 83333)
Host Organism:
Method Details:
Experimental Method:
Resolution:
3.10 Å
Aggregation State:
PARTICLE
Reconstruction Method:
SINGLE PARTICLE