Deposition Date
2019-03-13
Release Date
2019-06-19
Last Version Date
2024-10-30
Entry Detail
PDB ID:
6JMR
Keywords:
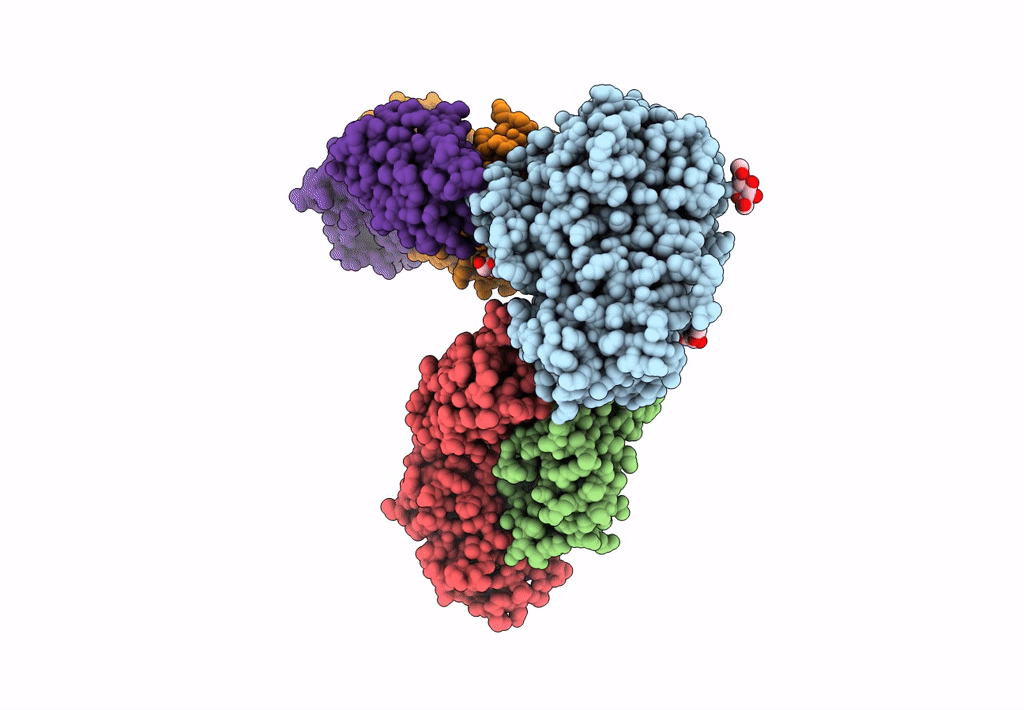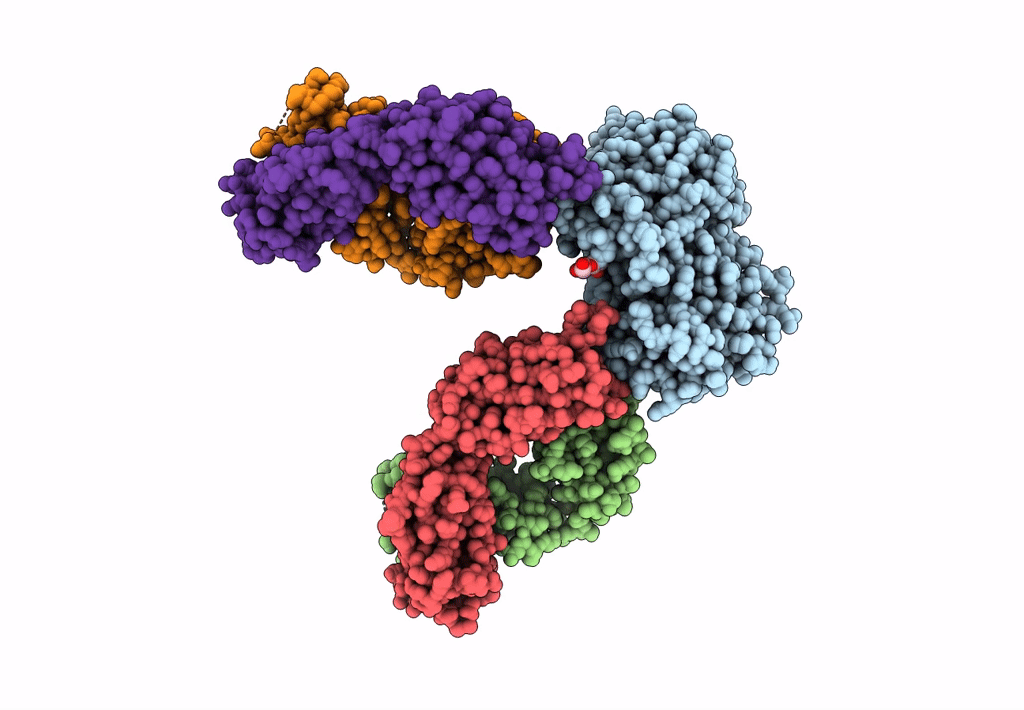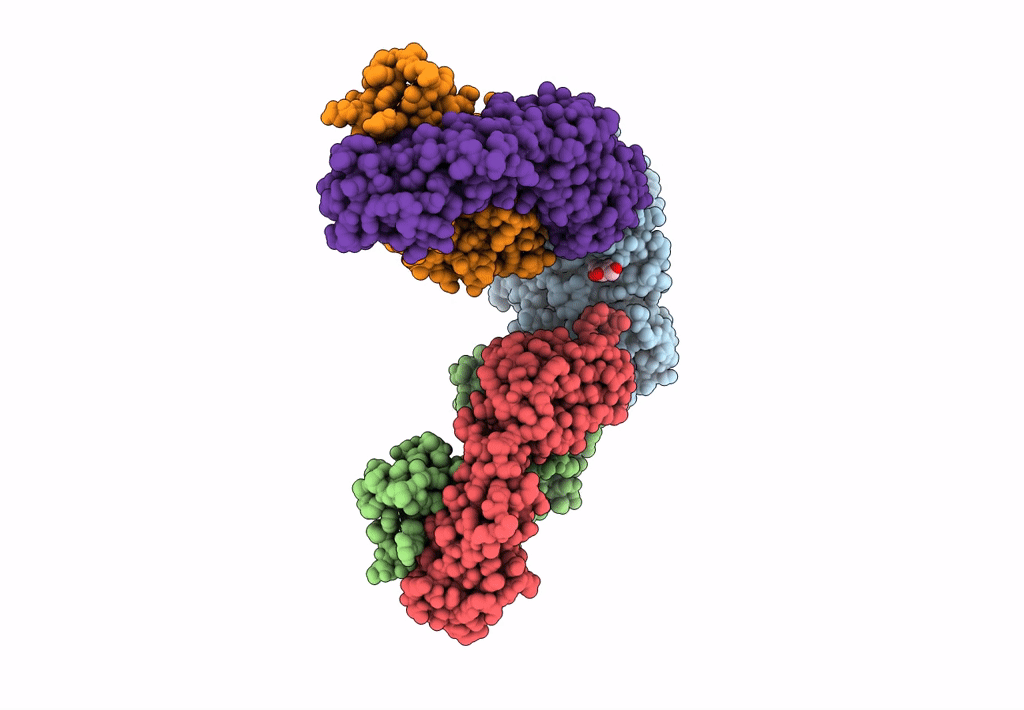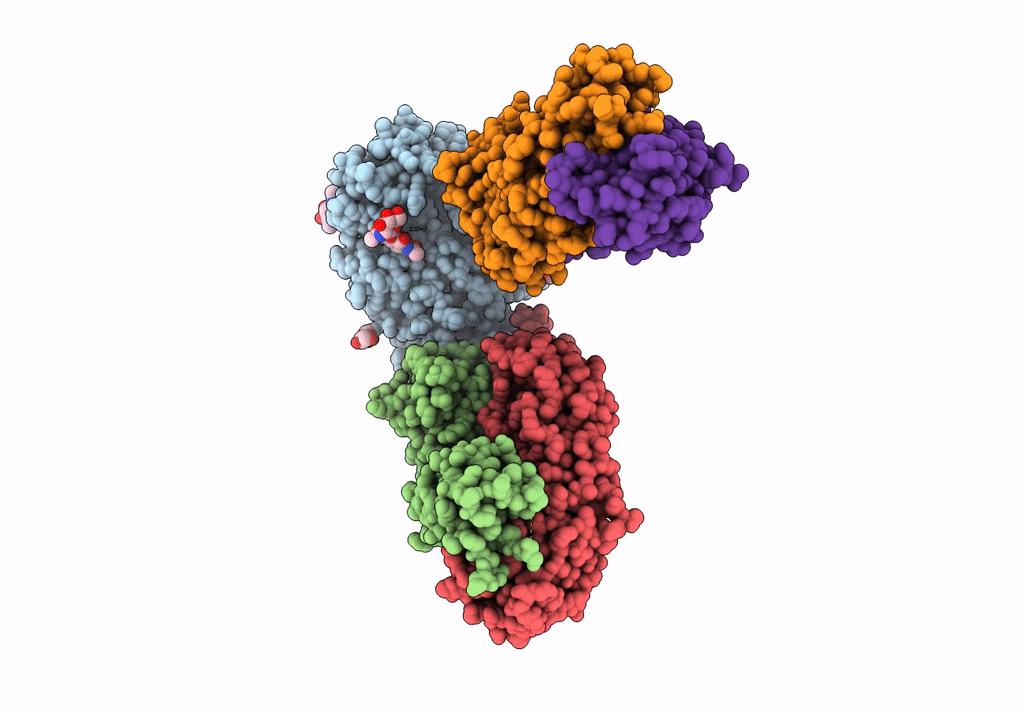
Title:
CD98hc extracellular domain bound to HBJ127 Fab and MEM-108 Fab
Biological Source:
Source Organism(s):
Mus musculus (Taxon ID: 10090)
Homo sapiens (Taxon ID: 9606)
Homo sapiens (Taxon ID: 9606)
Expression System(s):
Method Details:
Experimental Method:
Resolution:
4.10 Å
Aggregation State:
PARTICLE
Reconstruction Method:
SINGLE PARTICLE


