Deposition Date
2019-03-03
Release Date
2019-12-18
Last Version Date
2023-11-22
Entry Detail
PDB ID:
6JKY
Keywords:
Title:
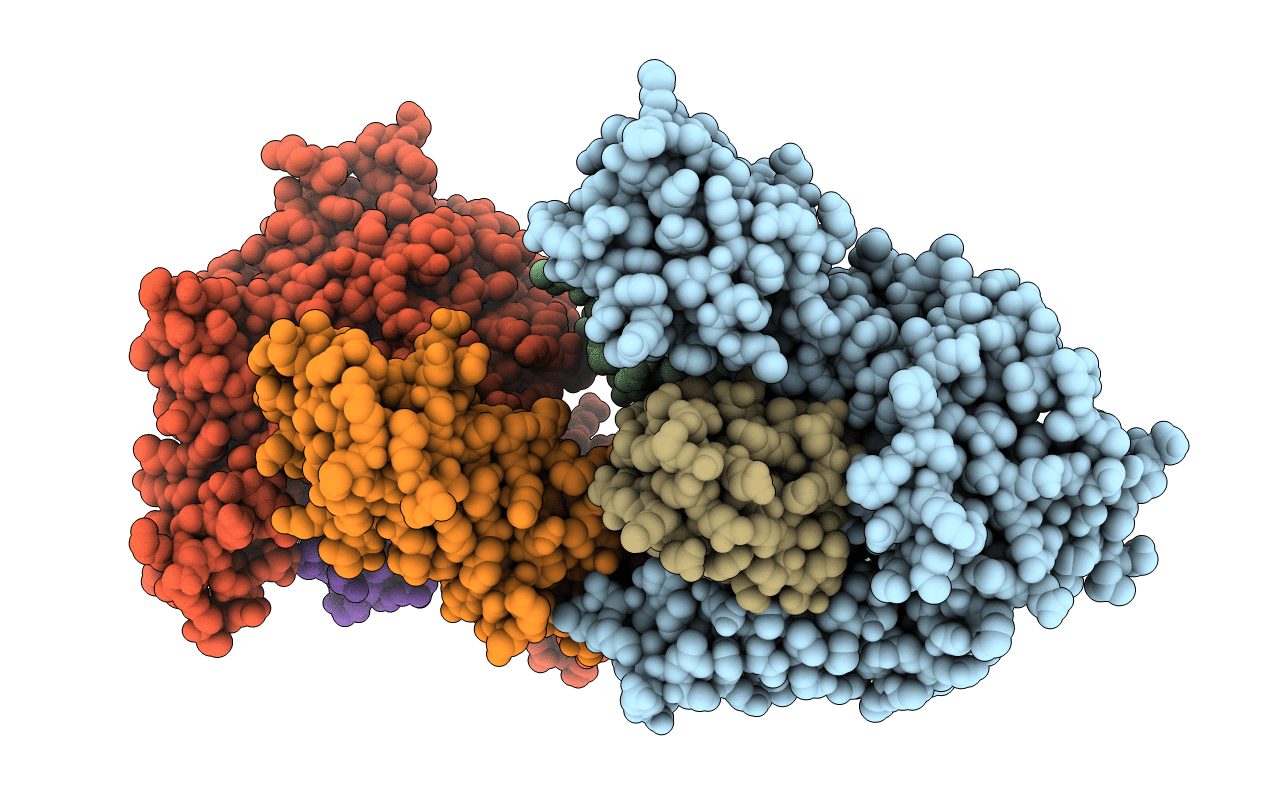
Crystal structure of MvcA-UBE2N-Ub complex from Legionella pneumophila
Biological Source:
Source Organism:
Legionella pneumophila subsp. pneumophila str. Philadelphia 1 (Taxon ID: 272624)
Homo sapiens (Taxon ID: 9606)
Schistosoma margrebowiei (Taxon ID: 48269)
Homo sapiens (Taxon ID: 9606)
Schistosoma margrebowiei (Taxon ID: 48269)
Host Organism:
Method Details:
Experimental Method:
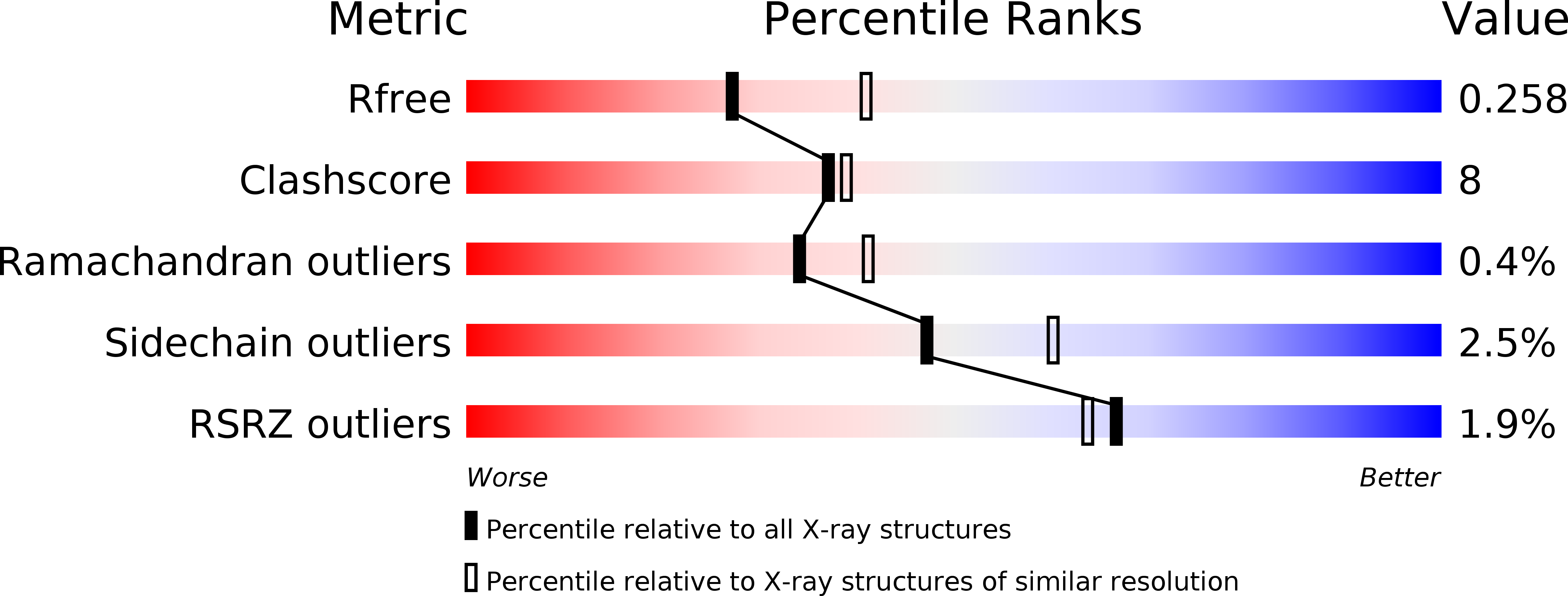
Resolution:
2.45 Å
R-Value Free:
0.25
R-Value Work:
0.20
R-Value Observed:
0.21
Space Group:
C 2 2 21