Deposition Date
2019-02-14
Release Date
2019-04-17
Last Version Date
2024-10-23
Entry Detail
PDB ID:
6JGJ
Keywords:
Title:
Crystal structure of the F99S/M153T/V163A/E222Q variant of GFP at 0.78 A
Biological Source:
Source Organism:
Aequorea victoria (Taxon ID: 6100)
Host Organism:
Method Details:
Experimental Method:
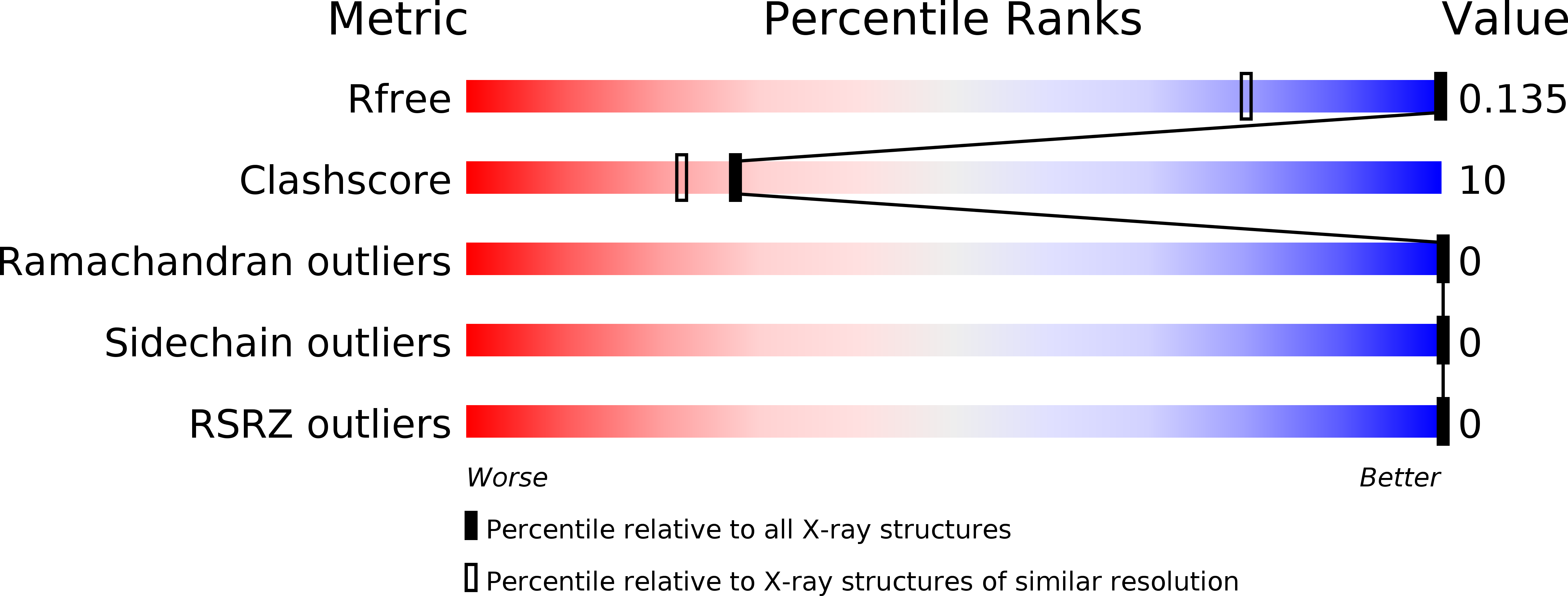
Resolution:
0.78 Å
R-Value Free:
0.12
R-Value Work:
0.10
Space Group:
P 21 21 21