Deposition Date
2018-10-09
Release Date
2019-07-03
Last Version Date
2024-10-23
Entry Detail
PDB ID:
6IJC
Keywords:
Title:
Structure of MMPA-CoA dehydrogenase from Roseovarius nubinhibens ISM
Biological Source:
Source Organism(s):
Roseovarius nubinhibens ISM (Taxon ID: 89187)
Expression System(s):
Method Details:
Experimental Method:
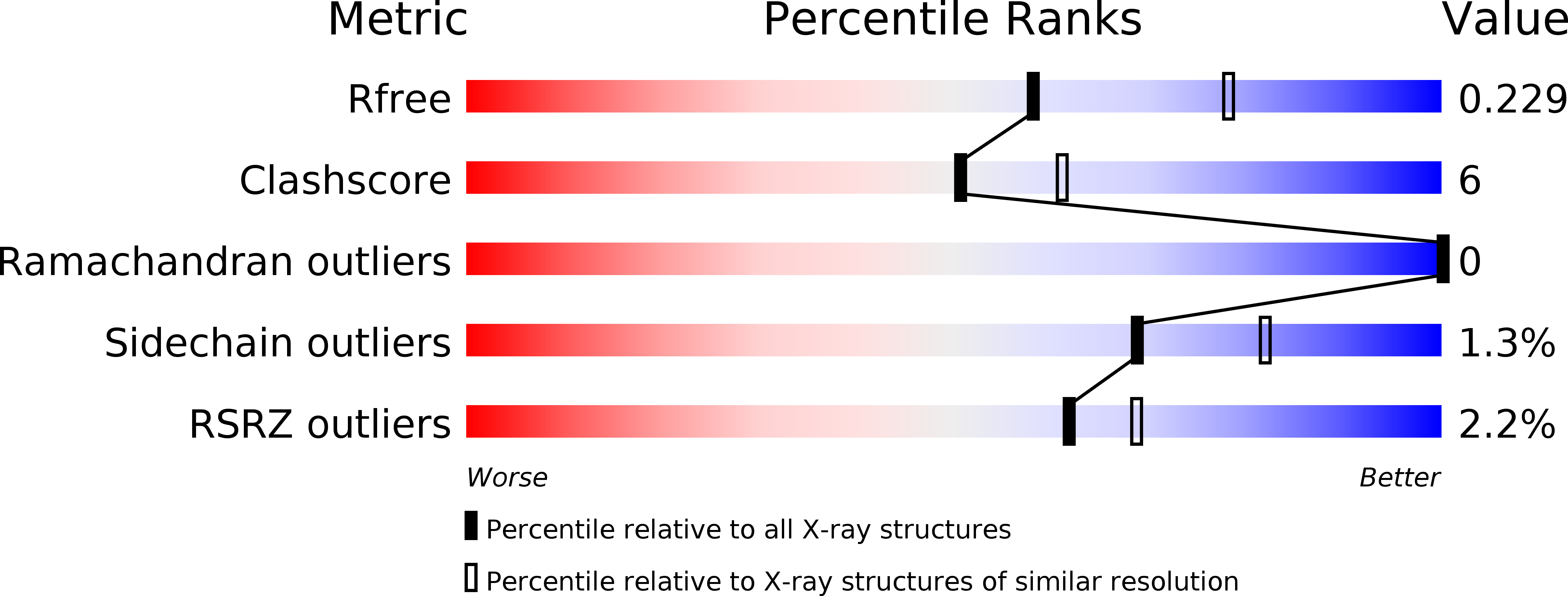
Resolution:
2.30 Å
R-Value Free:
0.22
R-Value Work:
0.17
R-Value Observed:
0.17
Space Group:
P 21 21 21