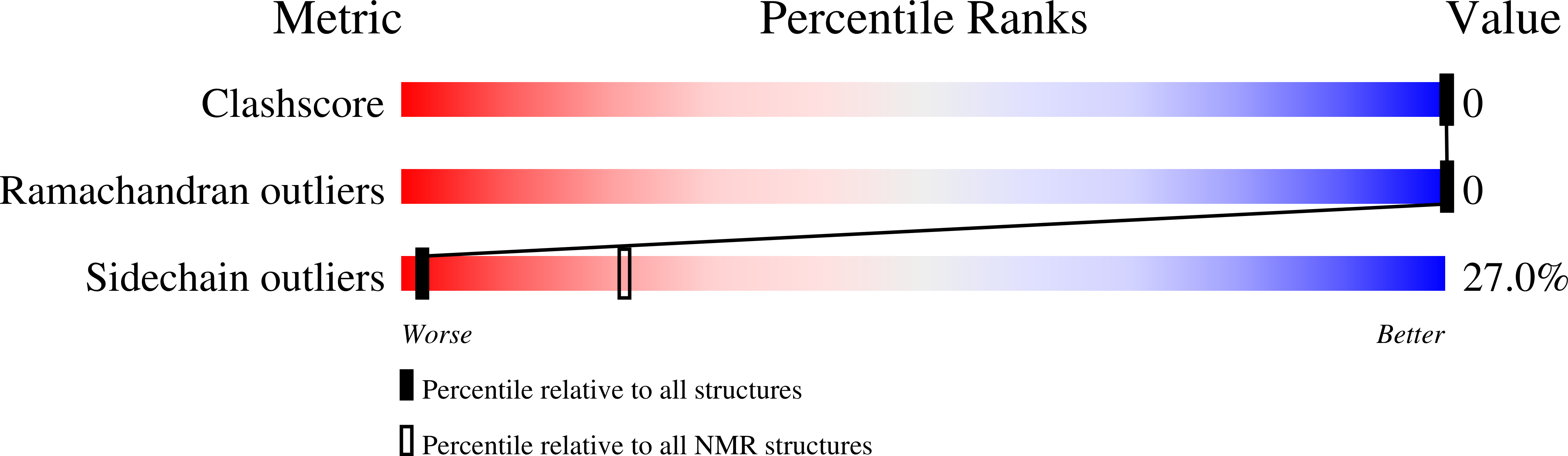
Deposition Date
2018-10-11
Release Date
2019-10-30
Last Version Date
2024-11-13
Entry Detail
PDB ID:
6HVK
Keywords:
Title:
Pepducin UT-Pep2 a biased allosteric agonist of Urotensin-II receptor
Biological Source:
Source Organism:
Homo sapiens (Taxon ID: 9606)
Method Details:
Experimental Method:
Conformers Calculated:
100
Conformers Submitted:
10
Selection Criteria:
structures with the lowest energy