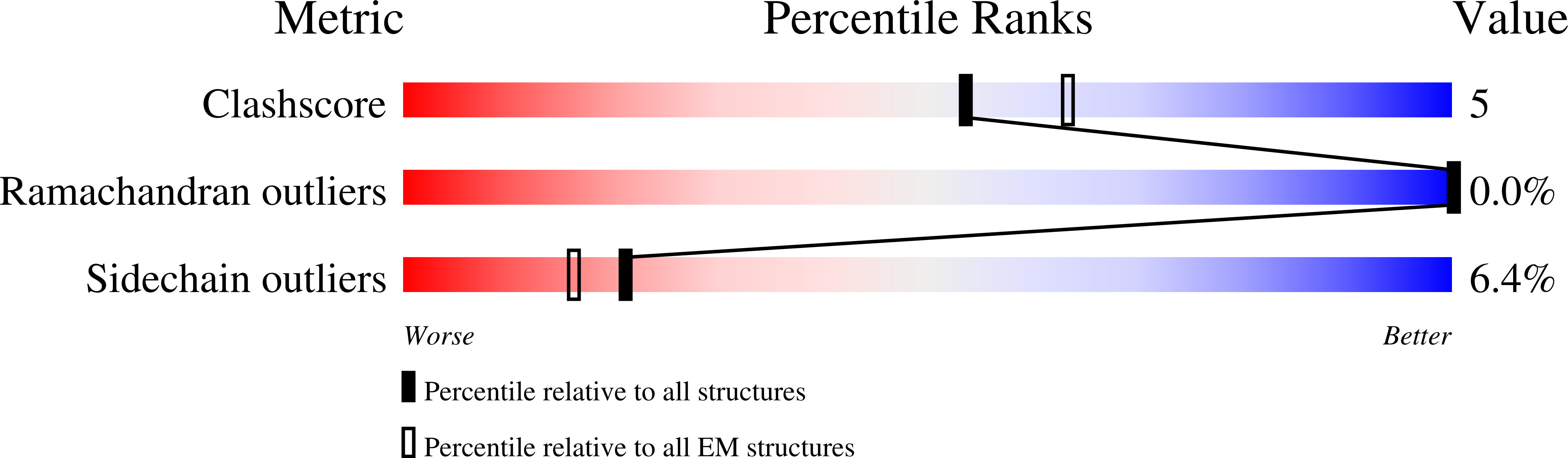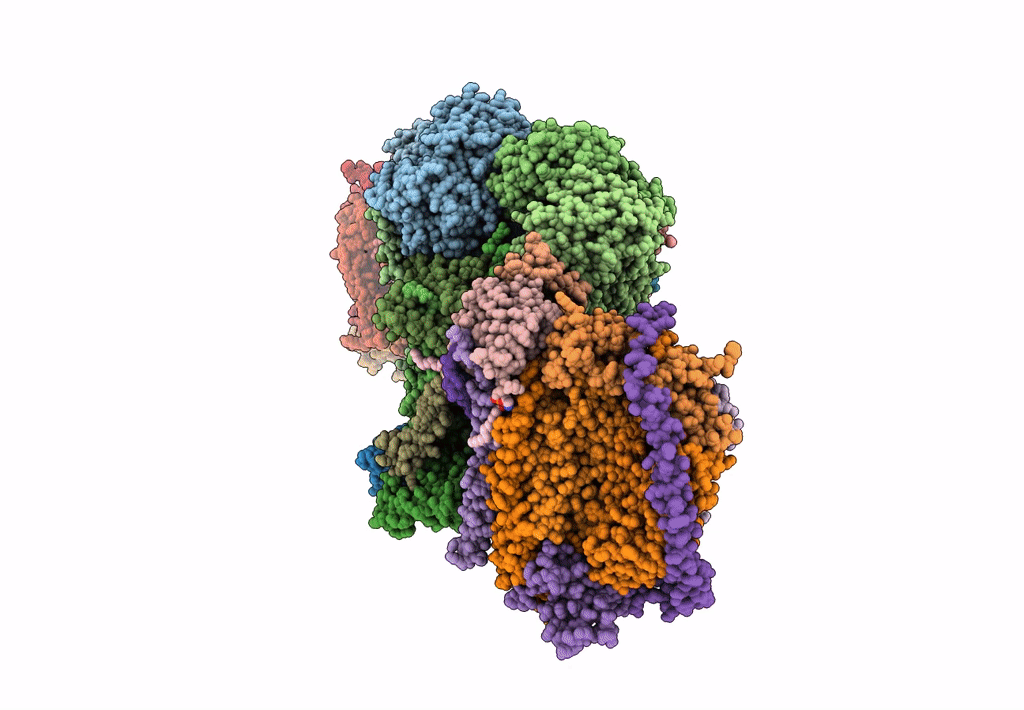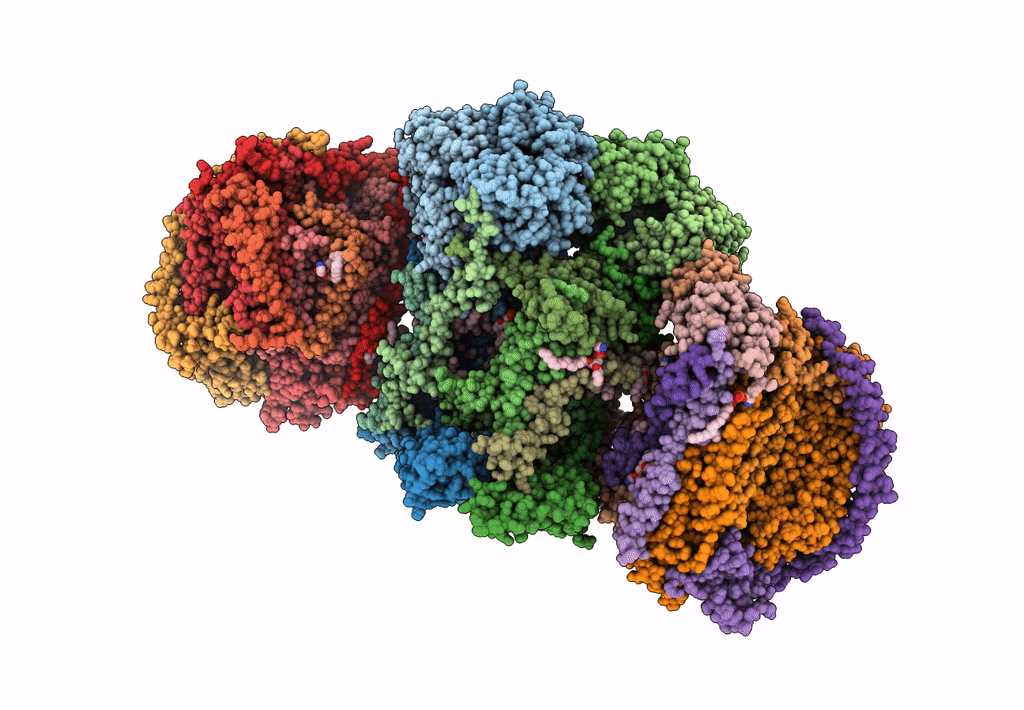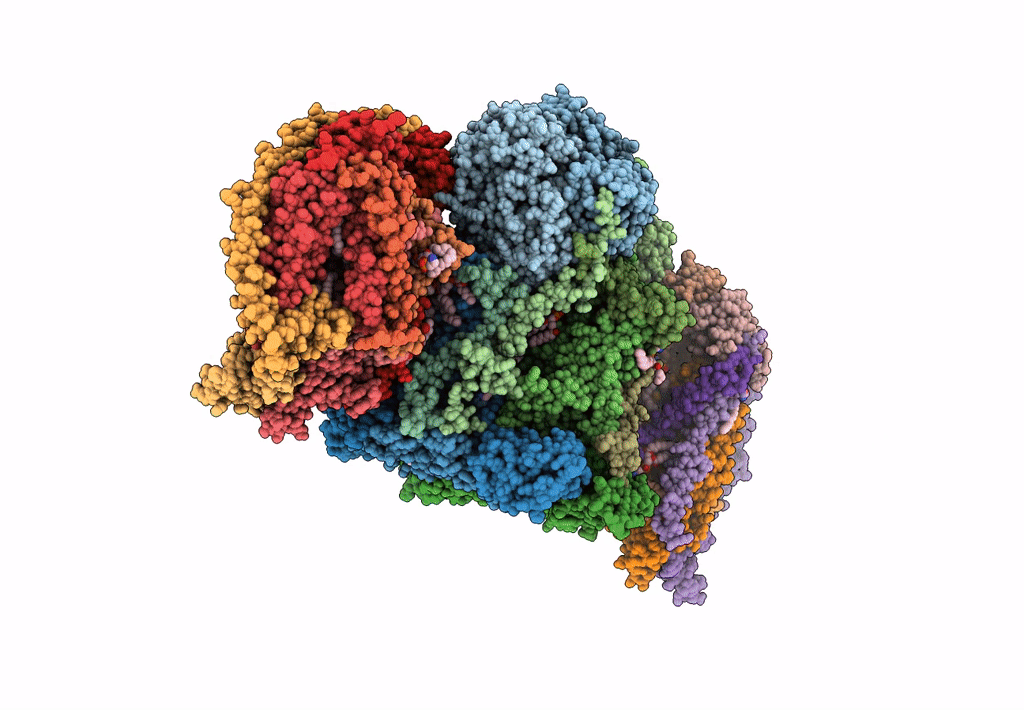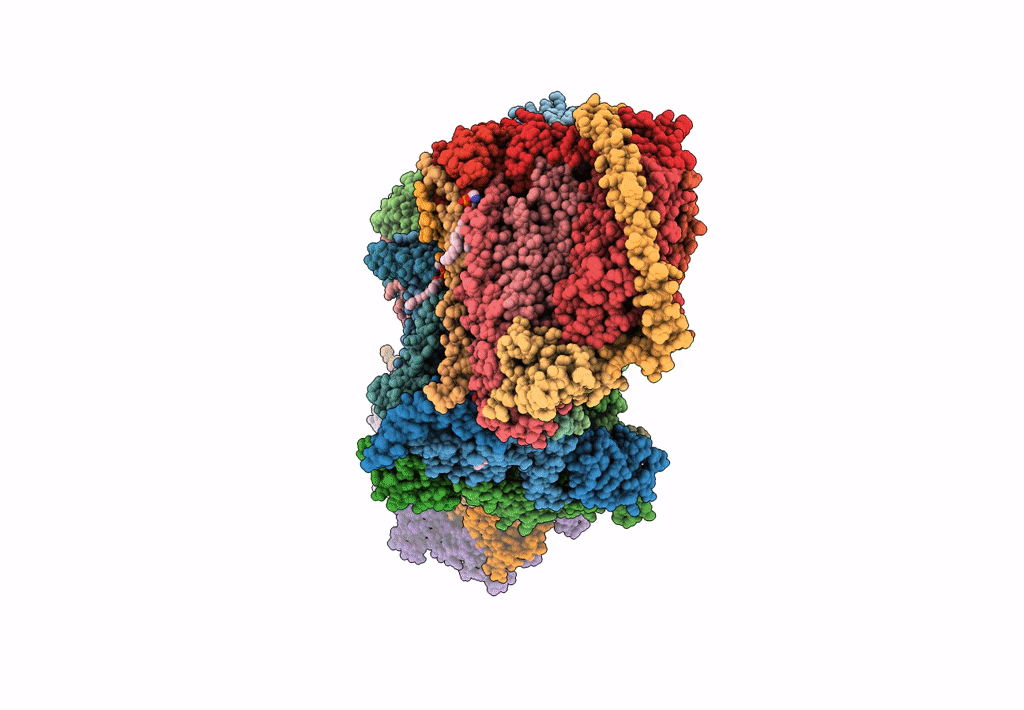
Deposition Date
2018-10-05
Release Date
2018-12-26
Last Version Date
2024-11-20
Entry Detail
PDB ID:
6HU9
Keywords:
Title:
III2-IV2 mitochondrial respiratory supercomplex from S. cerevisiae
Biological Source:
Source Organism(s):
Expression System(s):
Method Details:
Experimental Method:
Resolution:
3.35 Å
Aggregation State:
PARTICLE
Reconstruction Method:
SINGLE PARTICLE