Deposition Date
2018-08-17
Release Date
2019-08-28
Last Version Date
2024-01-17
Entry Detail
PDB ID:
6HD0
Keywords:
Title:
Common mode of remodeling AAA ATPases p97/CDC48 by their disassembly cofactors ASPL/PUX1
Biological Source:
Source Organism(s):
Homo sapiens (Taxon ID: 9606)
Arabidopsis thaliana (Taxon ID: 3702)
Arabidopsis thaliana (Taxon ID: 3702)
Expression System(s):
Method Details:
Experimental Method:
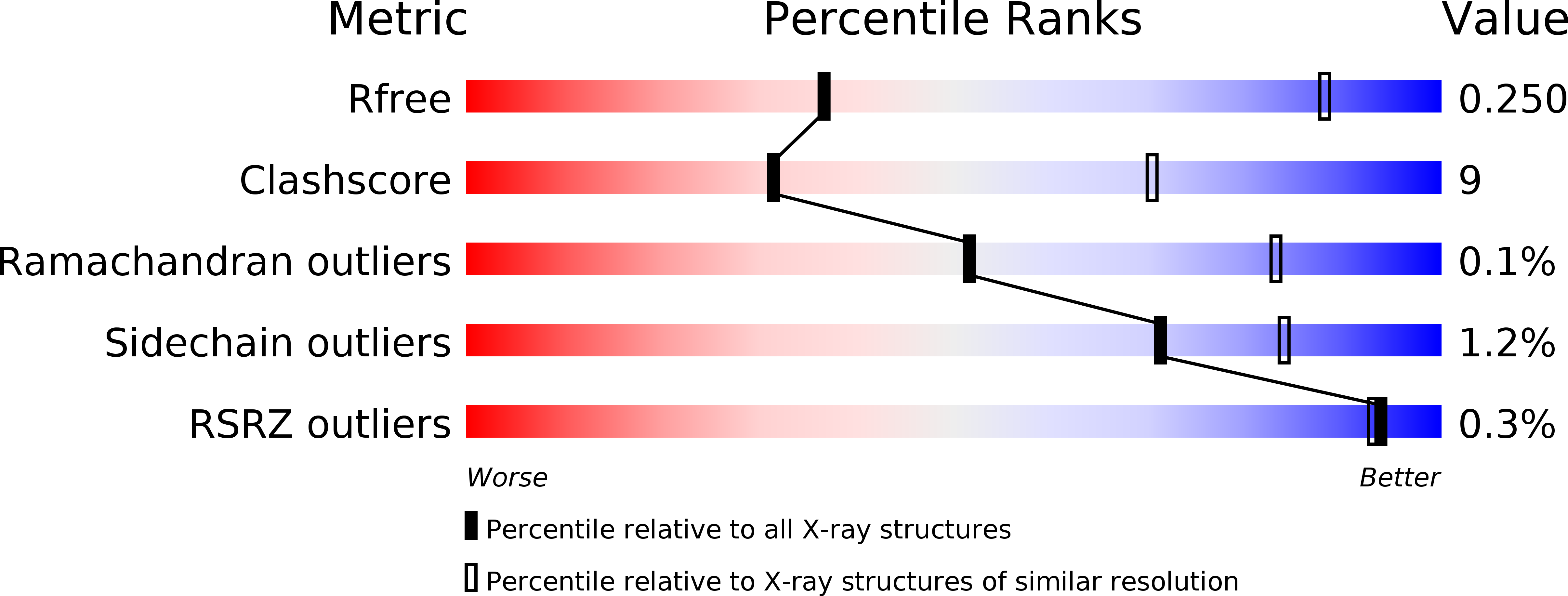
Resolution:
3.73 Å
R-Value Free:
0.25
R-Value Work:
0.20
R-Value Observed:
0.21
Space Group:
P 31