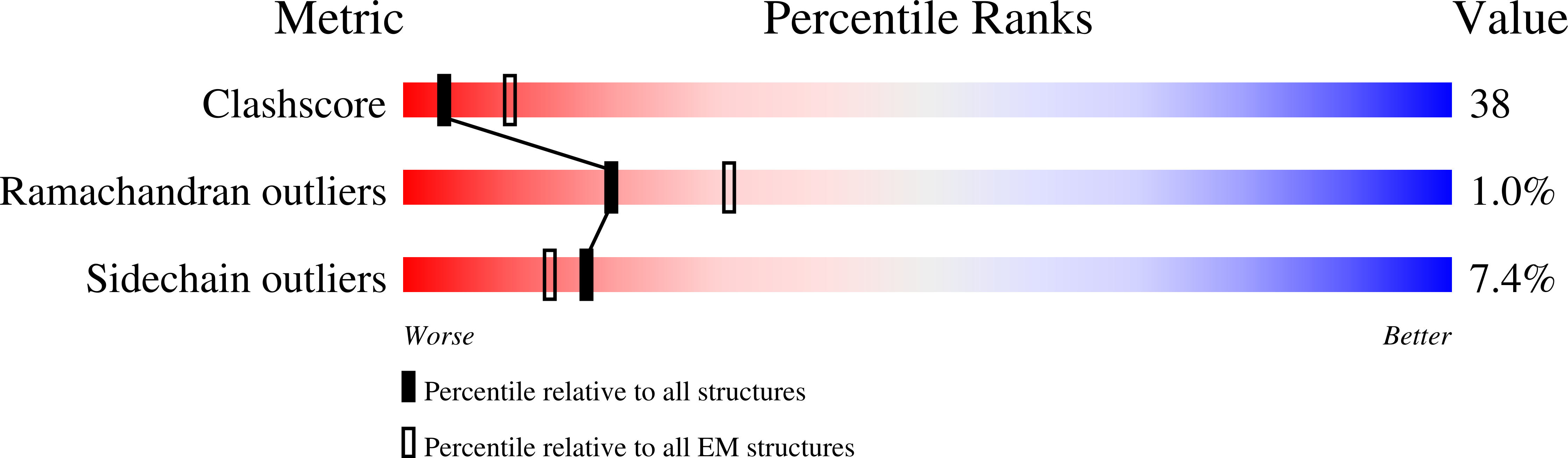Deposition Date
2018-08-03
Release Date
2020-02-12
Last Version Date
2024-05-15
Entry Detail
PDB ID:
6H9G
Keywords:
Title:
Influenza A nucleoprotein docked into 3D helical structure of the wild type ribonucleoprotein complex obtained using cryoEM. Conformation 1.
Biological Source:
Source Organism(s):
Method Details:
Experimental Method:
Resolution:
11.00 Å
Aggregation State:
HELICAL ARRAY
Reconstruction Method:
HELICAL