Deposition Date
2018-06-19
Release Date
2019-05-29
Last Version Date
2024-11-06
Entry Detail
PDB ID:
6GUT
Keywords:
Title:
CRYSTAL STRUCTURE OF NON-TYPEABLE HAEMOPHILUS INFLUENZAE PROTEIN E AND PILA EXPRESSED AS A SINGLE-CHAIN CHIMERIC PROTEIN
Biological Source:
Source Organism(s):
Haemophilus influenzae (Taxon ID: 727)
Expression System(s):
Method Details:
Experimental Method:
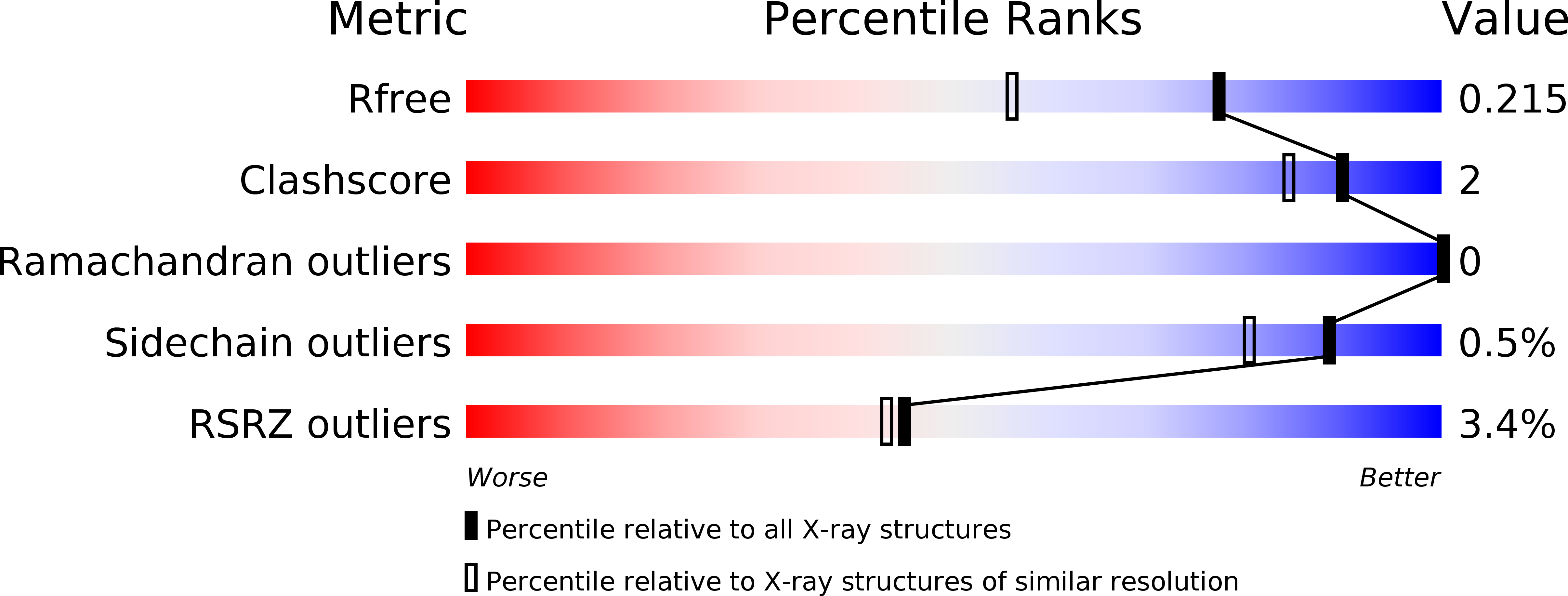
Resolution:
1.63 Å
R-Value Free:
0.20
R-Value Work:
0.17
R-Value Observed:
0.17
Space Group:
C 1 2 1