Deposition Date
2018-06-01
Release Date
2018-07-25
Last Version Date
2024-10-16
Entry Detail
PDB ID:
6GOK
Keywords:
Title:
X-ray structure of the adduct formed upon reaction of bovine pancreatic ribonuclease with a Pd(II) complex bearing N,N-pyridylbenzimidazole derivative with an alkylated sulphonate side chain
Biological Source:
Source Organism(s):
Bos taurus (Taxon ID: 9913)
Method Details:
Experimental Method:
Resolution:
2.65 Å
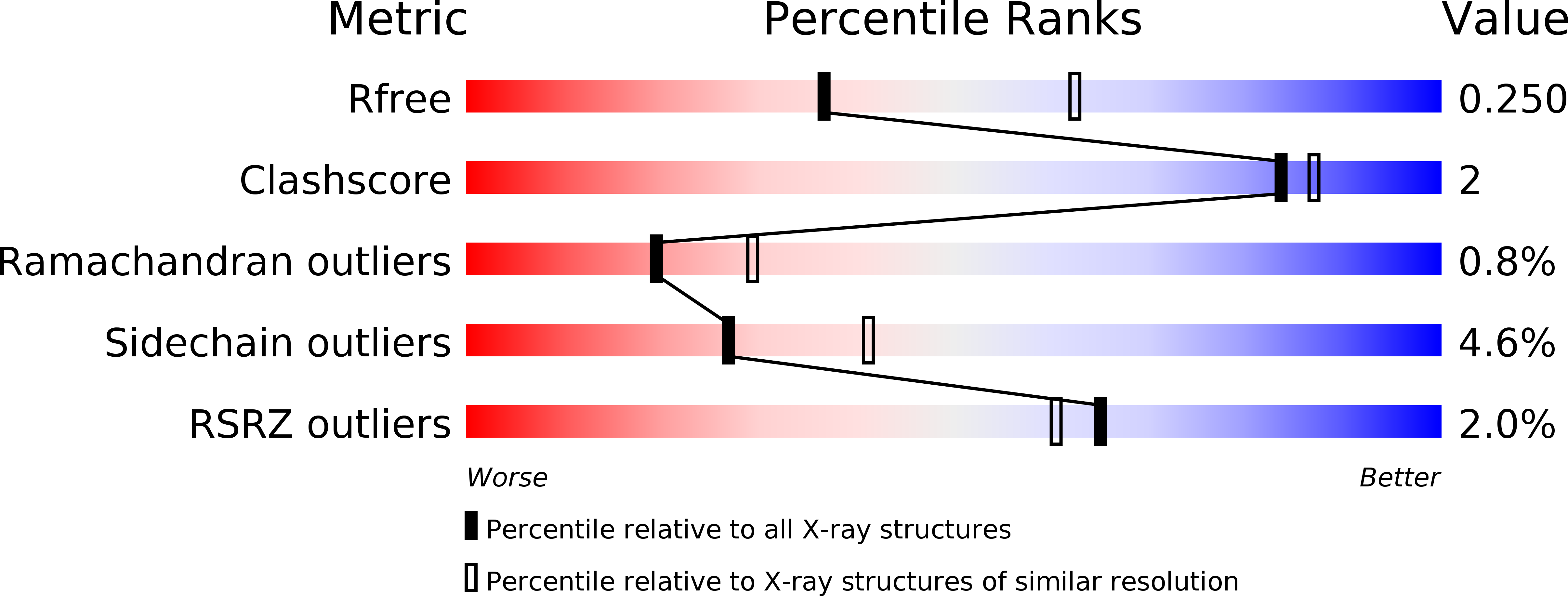
R-Value Free:
0.25
R-Value Work:
0.18
R-Value Observed:
0.19
Space Group:
C 1 2 1