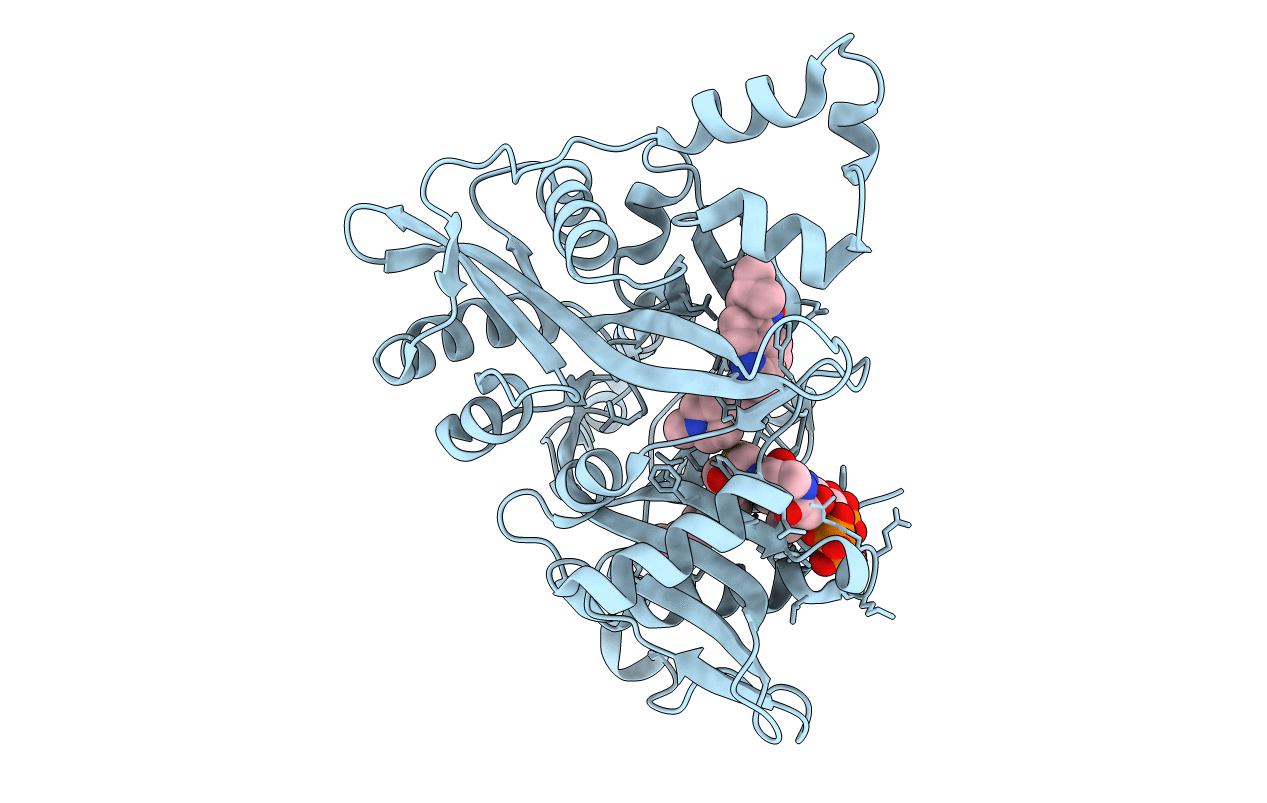
Deposition Date
2018-05-31
Release Date
2018-09-26
Last Version Date
2024-01-17
Entry Detail
PDB ID:
6GNU
Keywords:
Title:
Crystal Structure of Leishmania major N-Myristoyltransferase (NMT) With Bound Myristoyl-CoA and a Quionlinyl aryl sulphonamide ligand
Biological Source:
Source Organism:
Leishmania major (Taxon ID: 5664)
Host Organism:
Method Details:
Experimental Method:
Resolution:
1.54 Å
R-Value Free:
0.18
R-Value Work:
0.15
R-Value Observed:
0.15
Space Group:
P 1 21 1


