Deposition Date
2018-04-26
Release Date
2018-09-19
Last Version Date
2024-10-23
Entry Detail
PDB ID:
6GEG
Keywords:
Title:
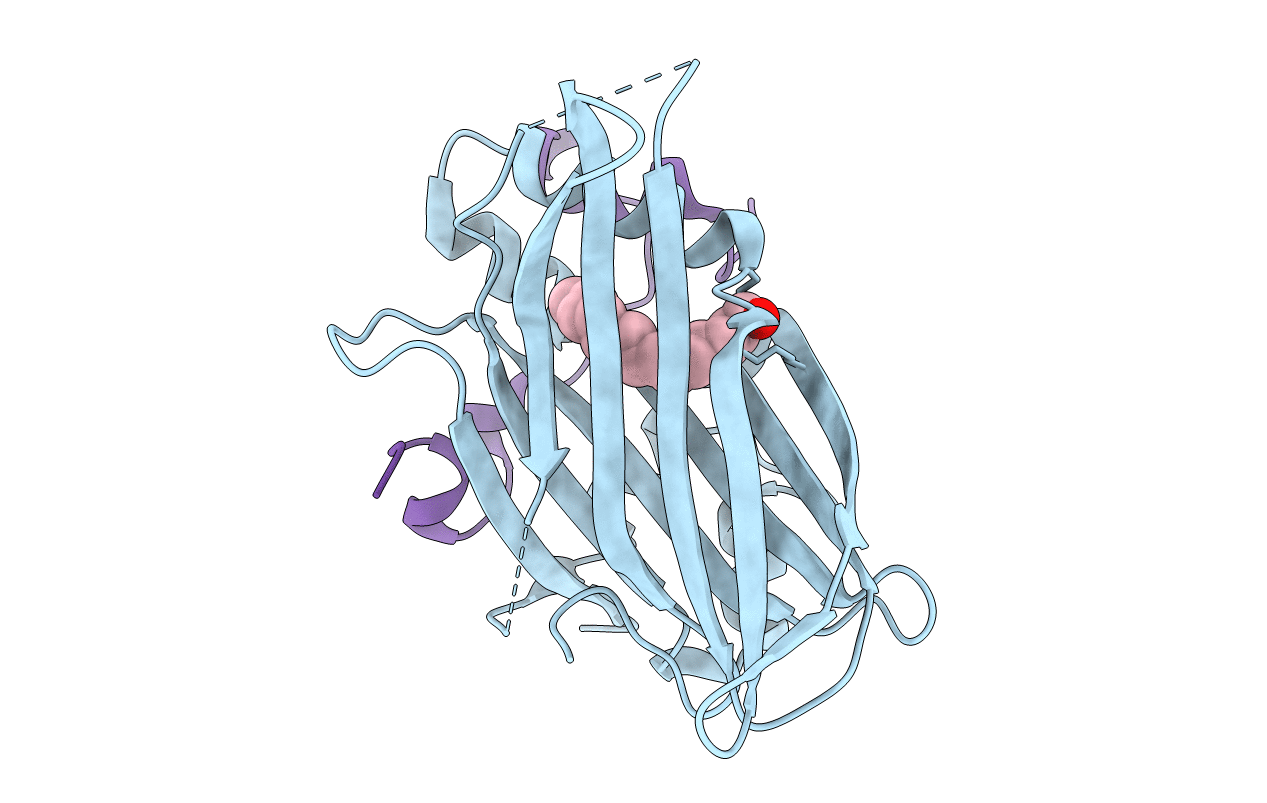
TEAD4 (216-434);Y429F COMPLEXED WITH YAP PEPTIDE (60-100); S94A AND MYRISTOATE (COVALENTLY BOUND) AT 2.23A (P41212 CRYSTAL FORM); MYRISTOYLATION WAS DONE BY ADDING MYR-COA
Biological Source:
Source Organism(s):
Homo sapiens (Taxon ID: 9606)
Expression System(s):
Method Details:
Experimental Method:
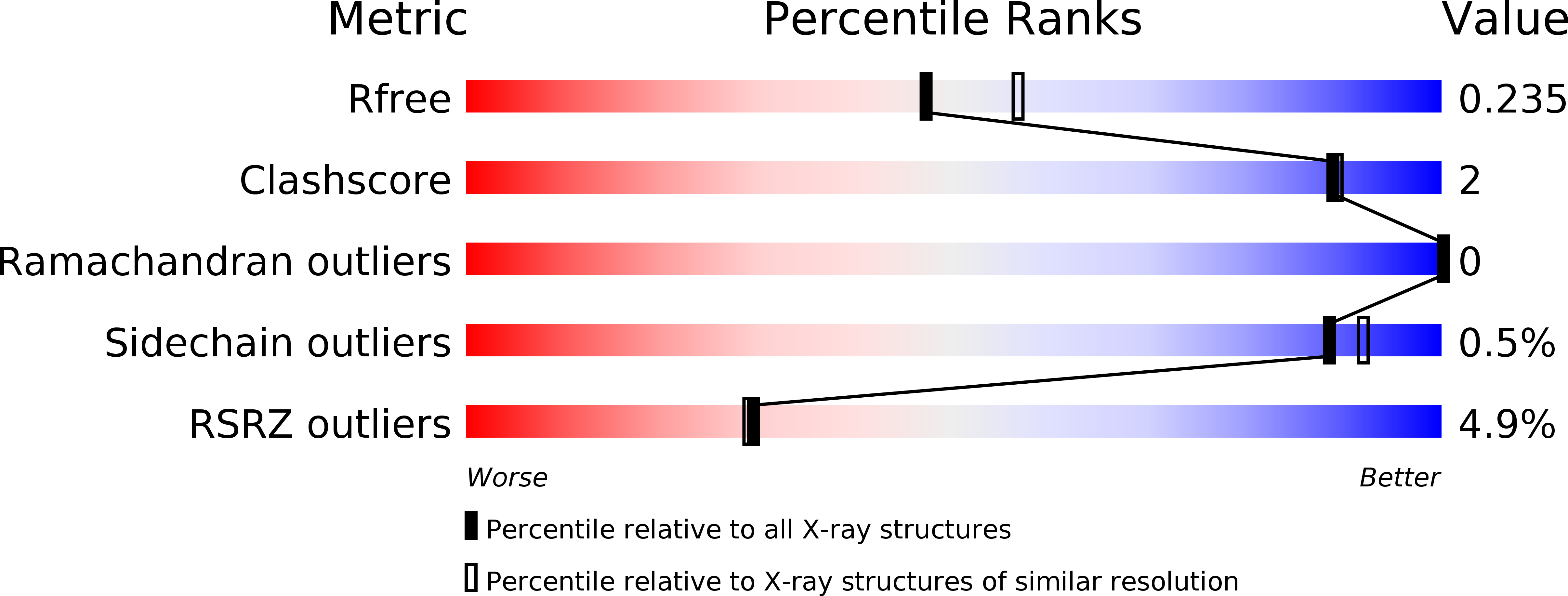
Resolution:
2.23 Å
R-Value Free:
0.23
R-Value Work:
0.20
R-Value Observed:
0.20
Space Group:
P 41 21 2