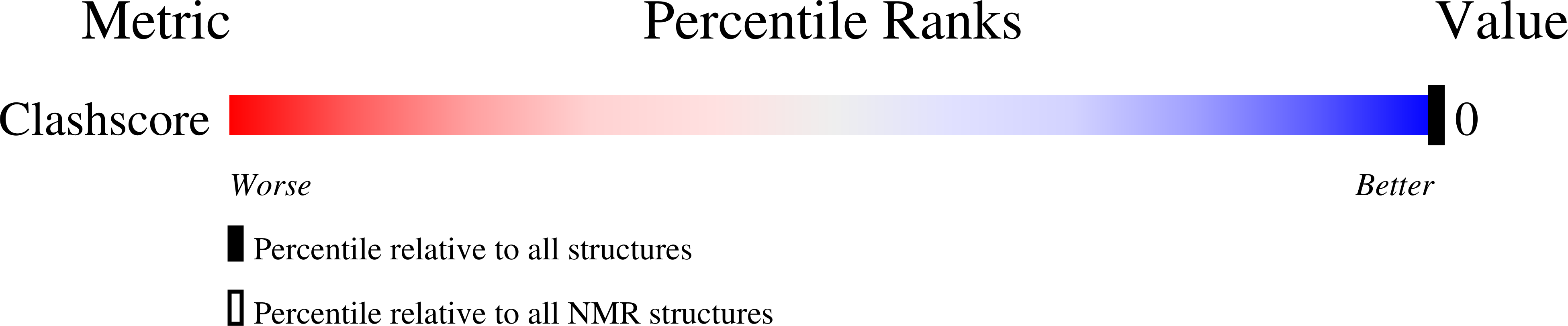Deposition Date
1997-11-07
Release Date
1998-01-28
Last Version Date
2024-05-22
Entry Detail
PDB ID:
6GAT
Keywords:
Title:
SOLUTION NMR STRUCTURE OF THE L22V MUTANT DNA BINDING DOMAIN OF AREA COMPLEXED TO A 13 BP DNA CONTAINING A TGATA SITE, REGULARIZED MEAN STRUCTURE
Biological Source:
Source Organism(s):
Emericella nidulans (Taxon ID: 162425)
Expression System(s):
Method Details:
Experimental Method:
Conformers Calculated:
34
Conformers Submitted:
1
Selection Criteria:
REGULARIZED MEAN STRUCTURE